Keywords
INTRODUCTION
Postimplantation syndrome (PIS) is a condition frequently observed following endovascular treatment of aortic disease. It is believed to result from complex interactions between the vascular endothelium and the fabric of the endoprosthesis. Clinical signs include significant leukocytosis, fever, elevated C-reactive protein (CRP), decreased platelet counts, and/or coagulation disturbances. Potential theoretical mechanisms of PIS are extensive local endothelial activation following stent application, contact activation of blood components by the graft fabric, and thrombus adhesion. Consecutive release of cytokines and arachidonic acid metabolites, together with leukocyte activation, may explain the systemic symptoms.1-3 Because the issue of prophylactic antibiotics to be given routinely with endograft therapy is yet to be settled4-8 and since even the recommendations given were nebulous,9 the need for prolonged antibiotics was a subject of interest.
Therefore, this prospective randomized pilot trial was designed to compare the influence of perioperative single-shot treatment versus prolonged (7 days) antibiotic therapy on parameters of infection and PIS after thoracic endografting, as assessed by clinical and laboratory findings.
METHODS
After approval of the study by the local ethics committee, the patients were screened and randomized for this trial prior to stent-graft repair of an aortic abnormality. After giving their informed consent, the patients were randomized to one of 2 groups using the closed envelope method, to receive either periprocedural (over 24 hours, group 1) or prolonged (over 7 days, group 2) antibiotic therapy (cefuroxime, 20 mg/kg 3 times per day intravenously). The demographic characteristics of the 2 groups are shown in Table 1. Exclusion criteria were impaired renal or hepatic function, acute or chronic inflammatory conditions like pneumonia, or urinary tract or catheter infection. Further exclusion criteria were blood transfusion and prior administration of steroids and nonsteroidal anti-inflammatory agents.
Based on multislice computed tomography or magnetic resonance images, stent-grafts were deployed in a hybrid laboratory with catheterization and imaging capabilities, including digital angiography. General anesthesia was initiated with midazolam (0.1 mg kg-1) administered 2 minutes prior to induction with etomidate (0.3 mg kg-1) and sufentanil (0.5 µg kg-1), and then maintained with propofol at an infusion rate of 80 to 120 µg/kg-1min-1. Neuromuscular blockade was achieved with rocuronium (0.65 mg kg-1). Patients were ventilated to normocapnia monitored by end-tidal carbon dioxide (PETCO2), with fractional oxygen of 0.3 to 0.4. All the patients were equipped with a central venous line. The right radial artery was used for continuous recordings of arterial pressure and all the patients received 5000 units of unfractionated heparin. The femoral artery was surgically exposed to accommodate a 24-F stent-graft introducer system. Using the Seldinger technique, a stiff 260-cm, 0.89-mm (0.035-inch) wire (Back-up Meier wire [Boston Scientific Inc., Ratingen, Germany]) was placed over a pigtail catheter in the true lumen, under both fluoroscopic and transesophageal ultrasound guidance. Carefully advanced over the stiff wire, placement of customized stent-grafts (Medtronic Talent or Valiant) was performed under rapid right ventricular pacing to avoid misplacement.10 Short inflation of a latex balloon to improve stent apposition was optional. The final result (closure of the proximal entry or exclusion of the penetrating aortic ulcers) was documented by contrast fluoroscopy and ultrasound. After removing sheath and guide wire, the access site was closed surgically and the patients were transferred to the intensive care unit for extubation and monitoring. During follow-up, the radial artery and central venous lines were removed. The antibiotics were infused over a peripheral venous access. We collected serial peripheral venous blood samples and body temperature measurements prior to the intervention and 12, 24, and 48 hours and 3, 5, and 7 days after stent-graft deployment. Laboratory analyses included CRP, white blood counts (WBC) and procalcitonin (PCT). The rationale for using PCT was based on its high sensitivity for obtaining an infectious etiology in light of a value greater than 0.05 ng/mL. Blood samples for both aerobic and anaerobic culturing were obtained prior to the intervention and 48 hours and 7 days afterwards by careful venipuncture. During the hospital stay, clinical and laboratory evaluation was performed to identify acute infections of the urinary tract and both lungs, as well as catheter infection. Patients with these infections were excluded from the study. Clinical and imaging follow-up was performed at 3 and 12 months and annually after the first year in all patients.
Statistical Analysis
The primary endpoint of our trial was the increase and difference in CRP, white blood count, PCT, and body temperature. For the statistical analysis of intragroup differences over time, the Wilcoxon rank test was used. The Mann-Whitey test was performed for comparison between 2 treatment groups at any given time. A P value less than .05 was considered significance. The descriptive statistical characteristics for quantitative parameters are listed as numbers (n), arithmetic mean (mean), median (med), minimum (min), maximum (max), and relative frequency (%). The arithmetic mean is expressed with the standard deviation. All data were processed using EXCEL and SPSS 15.0.
RESULTS
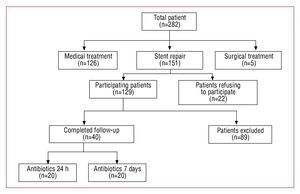
From October 2002 to October 2006, 282 patients with type B aortic dissection and penetrating aortic ulcer were screened. Due to the anatomic and clinical constellation, with no signs of malperfusion, no critical diameter of more than 6 cm and absence of pain in light of stable hemodynamic, 126 patients were treated medically, with close clinical and imaging surveillance. Five patients with an extended type B dissection, signs of peripheral malperfusion and retrograde progression into the ascending aorta were treated surgically. The remaining 151 were treated by endovascular stent-graft repair. Twenty-two patients refused to participate in this prospective pilot trial, while 129 patients were assigned to the trial. During in-hospital follow-up, 89 were excluded from analysis due to pneumonia (n=13), anemia with blood transfusion (n=31), renal failure (n=17), central venous catheter infection (n=7), urinary catheter infection (n=18), or in-hospital mortality (n=3), while 40 patients with aortic syndromes (16 men and 4 women, aged 46 to 64 years [mean 54 (5) years] in group 1 and 18 men and 2 women, aged 45 to 69 years [mean 56 (7) years] in group 2) completed the trial (Figure 1). There were no differences between the 2 groups in terms of demographic parameters (Table 1). In addition, there were no differences with respect to the anatomic and procedural characteristics (Table 2); 13 patients from group 1 and 14 patients from group 2 presented with an acute aortic dissection (mean duration since onset of symptoms 3.5 [1.2] days vs 2.8 [1.3] days; not significant). In 3 patients from group 1 and 1 from group 2, the left subclavian artery was overstented, with collateral bloodstream via the circle of Willis; thus, head vessel debranching was not necessary. All procedures were technically successful, with no deaths, strokes or paraplegia in either group.
Figure 1.Study population screened from 2002 to 2006.
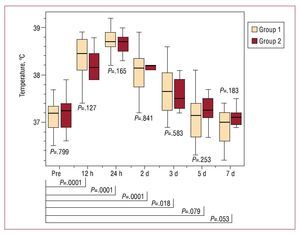
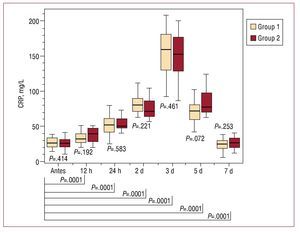
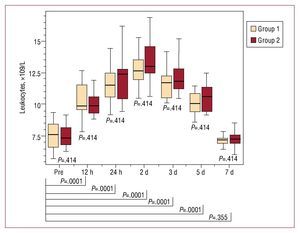
According to careful clinical monitoring, all the patients exhibited at least one criterion for PIS, including body temperature, CRP, PCT and/or elevated WBC, with no differences between the 2 groups (Figures 2-5). We recorded an elevation of both PCT and body temperature, with a maximum at 24 hours postintervention, an elevation of WBC, with a maximum after 48 hours, and a peak elevation of CRP at 72 hours in both groups; these alterations occurred between one and three days after stent-graft implantation in both. White blood cell counts and PCT normalized within seven days, while body temperature was normal within 5 days. C-reactive protein declined within seven days but failed to return to normal levels before discharge in both groups. Moreover, in both groups, all blood samples taken prior to the intervention and 48 hours and seven days afterwards were negative for aerobic and anaerobic bacteria after seven days in culture medium.
Figure. 2. Time course of the procalcitonin (PCT) values of group 1 (peri-interventional antibiotic treatment) and group 2 (prolonged antibiotic treatment) before and after stent-graft implantation. There is no statistically significant difference between the 2 groups at any time. After stent-graft implantation, there is a significant increase of PCT values during follow-up, with a maximum after 24 hours and a return to nonsignificant values after 7 days. Pre indicates preimplantation.
Figure 3. Time course of the body temperature of group 1 (peri-interventional antibiotic treatment) and group 2 (prolonged antibiotic treatment) before and after stent-graft implantation. There is no statistically significant difference between the 2 groups at any time. After stent-graft implantation there is a significant increase of body temperature during follow-up, with a maximum after 24 hours and a return to nonsignificant values after 5 days. Pre indicates preimplantation.
Figure 4. Time course of the C-reactive protein (CRP) values of group 1 (periinterventional antibiotic treatment) and group 2 (prolonged antibiotic treatment) before and after stent-graft implantation. There is no statistically significant difference between the 2 groups at any time. After stent-graft implantation, there is a significant increase of CRP values during follow-up, with a maximum after 3 days and no return to nonsignificant values within 7 days. Pre indicates preimplantation.
Figure. 5. Time course of the leukocyte values of group 1 (peri-interventional antibiotic treatment) and group 2 (prolonged antibiotic treatment) before and after stent-graft implantation. There is no statistically significant difference between the 2 groups at any time. After stent-graft implantation, there is a significant increase of leukocyte values during follow-up, with a maximum after 48 hours and a return to nonsignificant values after 7 days. Pre indicates preimplantation.
After a mean follow-up of 3.1 (1.7) years, the overall survival rate was 85.8% (3.9%) in group 1 and 87.1% (4.2%) in group 2 (P=.47). The aortic rupture-free survival rates were 93.3% (2.9%) versus 92.7% (3.1%), respectively (P=.83). During the serial image follow-up, the rates of false lumen thrombosis in the area of the stent-graft were 72% and 68% (P=.21) and thrombosis throughout the entire false lumen, 35% and 42%, respectively (P=.09). Repeat interventions due to leakage or dynamic obstruction of a side vessel were required in 15% and 20% of the cases (P=.13). The distributions to further stent-grafts and to surgical intervention were 10% versus 5% (P=.26) and 15% versus 5% (P=.06).
DISCUSSION
The PIS is a poorly defined, transient finding during the early postoperative phase following endovascular intervention with the use of stent-grafts, and is characterized by fever, leukocytosis, elevated CRP, decreased platelet counts and/or coagulation disturbances.11-15
In our series of 40 patients, we observed acute phase reaction in all the patients, with an increase in mean body temperature from 37.2 (0.3) °C prior to the intervention to 38.7 (0.3) °C 24 hours later, an elevation of WBC from 7.6 (1.0´109)/L to 12.7 (1.1´109)/L at 48 hours and an elevation of CRP from 10.4 (2.8 mg/L) to 157.4 (34) mg/L on day 3, whereas the blood samples taken prior to the intervention and 48 hours afterwards were negative for aerobic and anaerobic bacteria after seven days in culture medium. Most importantly, there were no differences in parameters related to PIS between the 2 groups. Moreover, we observed no differences between the 2 groups when acute and chronic type B dissections were compared. Due to the small number of patients with chronic type B dissections in our study, this aspect had to be confirmed in larger trials. Procalcitonin and body temperature reached their maximum after 24 hours, WBC after 48 hours and CRP after 72 hours in both groups, regardless of prolonged antibiotic therapy. Procalcitonin, body temperature, and white blood count returned to normal within the same time span even when antibiotics were given over 1 week. Only CRP values were mildly elevated 7 days after the intervention, with no differences between the 2 groups.
Fever has been reported after implantation of an endoprosthesis and has several potential etiologies, including bacterial translocation, possibly facilitated by transitory splanchnic ischemia, and the release of IL-6. Fever and leukocytosis after endovascular repair of aortic aneurysms was first noted by Blum et al in a multicenter series of 154 patients.13 The stent-grafts used in that study consisted of a nitinol frame covered with a thin, woven polyester fabric. In their series, 56% of the patients were found to have a body temperature of 38°C, and all the patients demonstrated leukocytosis, ranging from 9800 to 29 500 cells/dL. Similar observations have been reported since then, with fever present in up to 83% of the patients and leukocytosis in up to 58%.3,5,8,14,15-17 Experimental studies in pig, sheep, and canine models have suggested a local periaortic inflammatory response to endovascular exclusion of aortic aneurysms.18,19 In sheep subjected to endovascular implantation of heparin-coated Dacron-covered prostheses, macroscopic examination of the arterial wall one month after implantation revealed marked inflammatory perigraft responses with vascular wall thickening and adhesions around the grafts, and microscopic examination revealed a severe foreign-body response.19
To discriminate precisely between bacterial and nonbacterial inflammatory processes, PCT, as an early marker of bacterial infection, was also quantitatively assessed, but was similar in the 2 groups. The baseline concentration of PCT, a polypeptide that is produced in the liver and peripheral mononuclear cells, is <0.05 ng/mL.20,21 Under inflammatory conditions, such as sepsis or a single injection of endotoxin, serum PCT levels will rise.20,21 With a cutoff value over 0.5 ng/mL, sensitivity is 65% and specificity 96% for bacterial infection, with a negative predictive value of 98.8%.22,23 Regardless of prolonged antibiotics, we documented a rise in PCT from 0.03 (0.01) ng/mL to 0.15 (0.02) ng/mL 24 hours after the intervention, returning to a normal value within 7 days. Although PCT concentrations can increase to above normal in 32% of patients after minor and aseptic surgery, bacteria need not be present.24 Procalcitonin production can be induced by various stimuli, including trauma, tissue injury, and surgical cutdown to the access vessel. Therefore, the mild transient elevation of PCT is most likely explained by nonspecific cytokine liberation from the injured tissue, rather than by bacteremia, in the presence of sterile blood.25 Based on these considerations, patients with PIS and slightly elevated PCT despite sterile blood samples may benefit from single-shot antibiotics in combination with anti-inflammatory medication such as NSAID for a few days instead of prolonged antibiotics, an assumption that needs to be confirmed in further trials.
According to the experimental work of Burke et al in the early 1960s, the timing of antibiotics is of the utmost importance.26 Multiple studies over the following decade confirmed Burke's results and led to the current widespread practice of administering antibiotics just as the procedure is about to begin or within the last 2 hours before the intervention.27 If antibiotic prophylaxis is administrated more than 3 hours before the procedure, the incidence of adverse infectious events increases fivefold.28 While the timing of administration of the antibiotic has become fairly standard, the duration of administration remains debatable.
To the best of our knowledge, this analysis represents the first comparison of perioperative (24 hours) versus prolonged (7 days) prophylactic antibiotic treatment in the setting of aortic stent-graft placement. We could not demonstrate any short- or long-term advantage of prolonged antibiotic treatment beyond the day of the endovascular intervention with respect to preventing PIS. Based on observational results in a limited number of patients, prolonged use of antibiotics is not justified as a routine measure after endovascular intervention. This concept of single-shot antibiotics requires confirmation in larger randomized trials.
Limitations
One limitation of this trial is that, under such rare circumstances as isolated PIS after stent-graft placement for aortic abnormality, power calculation was not performed for sample size; thus, this trial represents just a pilot trial and, therefore, the results need to be confirmed in larger multicenter trials including power calculation. In addition, because of the exclusion of patients with infections of other causes, there is no presentation of an intention to treat approach.
ABBREVIATIONS
CRP: C-reactive protein
PCT: procalcitonin
PIS: postimplantation syndrome
Correspondence: C. A. Nienaber, MD, PhD, FACC, FESC, Department of Internal Medicine, Divisions of Cardiology, Pulmology, Intensive Care Medicine, University Hospital Rostock,
Rostock School of Medicine,
Ernst-Heydemann-Str. 6, 18057 Rostock, Germany
E-mail: christoph.nienaber@med.uni-rostock.de
Received April 2, 2009.
Accepted for publication July 14, 2009.