Keywords
INTRODUCTION
Chronic heart failure (HF) is a progressive disorder associated with a high mortality rate.1 In recent years, the existence of a complex interaction between hormonal, metabolic and nutritional changes, which could contribute to the progression of HF, has been demonstrated.2 In this respect, data surrounding the role of anabolic hormones is scarce and inconclusive: their levels are reduced in patients with HF and the appearance of an imbalance between anabolism and catabolism has been associated with the development of cachexia and a higher mortality during the progression of the disorder.3-7 In addition to this, anabolic deficiency in terms of free testosterone, dehydroepiandrosterone sulphate (DHEA-S) and insulin-like growth factor 1 (IGF-1) is frequent in men with HF and has a negative impact on their prognosis.3
Sex hormone-binding globulin (SHBG) is a plasma glycoprotein that binds to steroid sex hormones with high affinity, and which passively regulates its anabolic action by determining its free concentrations in plasma.8 Thus, high levels of SHBG result in increased binding to the hormone and a reduced free fraction, which, being biologically active, brings about reduced hormonal activity. Recently, SHBG has also been described as a multi-functional protein, capable of actively regulating the response to the steroid hormones and the entry of androgens in different cells and tissues.9-12 In addition to this, SHBG has a significant correlation with nutritional state; low levels are associated with central obesity and metabolic syndrome, while high levels occur with anorexia nervosa, protein-energy malnutrition and some forms of cancer.13-17 Low levels of SHBG have been associated with a higher cardiovascular risk in asymptomatic men.18,19 However, its significance is unknown in patients with HF.
Based on these data, our hypothesis was that SHBG could be of clinical signficance in patients with HF. The aim of this study was to examine the value of SHBG and other related hormones in male patients with HF, and its relation with cardiac death during the follow-up period.
METHODS
Study Population and Protocol
The study population consisted of 104 men with an established diagnosis of heart failure, consecutively enrolled in a specialist HF clinic over a period of 2 years (January 2003 to December 2004). The criteria for selection were: systolic dysfunction (left ventricular ejection fraction [LVEF] <40%) a stable clinical condition, defined as the patient not being admitted to hospital or showing signs of acute decompensation during the 4 previous weeks; and optimal medical treatment, remaining unchanged during at least the 4 previous weeks (100% angiotensin II converting enzyme inhibitors or angiotensin II receptor antagonists, 100% beta-blockers, 84% loop diuretics, 58% digoxin, 55% spironolactone). Due to the interaction between thyroid hormones and SHBG, patients with a history of thyroid disease or under treatment with thyroid hormones were excluded.20 Patients with current cancer or with a history of cancer were also excluded. Upon inclusion in the study, all clinical variables were gathered prospectively, and blood samples and a transthoracic echocardiography was carried out (Sonos 5500, Philips, Andover, Massachussets); the LVEF was calculated using the modified Simpson's rule, using second harmonic imaging. The study was approved by the local ethics committee and the informed consent of each patient was obtained. All of the patients were monitored for 3 years via visits to the clinic and telephone calls. The event studied was cardiac death, defined as secondary to refractory HF (including urgent cardiac transplantation) or occurring as sudden death unexplained by other causes. Monitoring of the patients was carried out via visits to the clinic, telephone calls and reviewing their medical records and clinical history.
Biochemical Parameters
A blood sample was taken upon inclusion in the study via puncture of the antecubital vein, following a fasting period of at least 12 hours and a rest period of 20 minutes. The plasma and the serum fraction were obtained following centrifugation at 2200 G for 20 minutes. The aliquots obtained were stored at -80ºC until the combined analysis. The levels of SHBG (nmol/L) were measured in duplicate in a specific radioimmune analysis test (Orion Diagnostica, Espoo, Finland), as were the levels of DHEA-S (µg/100mL) (Immunotech, Marseille, France), IGF-1 (ng/mL) (Immunotech, Marseille, France), cortisol (nmol/L) (Immunotech, Beckman Coulter company, Prague, Czech Republic) and tumour necrosis factor alpha (pg/ mL) (Biosource Europe S.A., Nivelles, Belgium). The levels of total testosterone, N-terminal pro B-type natriuretic peptide (NT-proBNP), free thyroxine and triiodothyronine were measured using an electrochemiluminescence immunoassay in a Modular E 170 analyser (Roche diagnostics, Mannheim, Germany). The serum levels of free testosterone were calculated with the validated equation of Vermeulen et al.21 The levels of C-reactive protein (mg/dL) were measured using an immunoturbidimetric test in a Modular P 800 analyser (Roche Diagnostics, Mannheim, Germany).
Statistical Analysis
Normal distribution of the studied variables was evaluated using the Kolmogorov-Smirnov test. Given the skewed distribution of SHBG, free testosterone, DHEA-S, LVEF, and NT-proBNP; these values were expressed as median [interquartile range] and for statistical analysis their natural logarithmic transformation was used. The quantitative variables with normal distribution were expressed as mean (SD), and the categorical variables as a number (percentage). The study of the determinants of SHBG levels of SHBG was carried out using Spearman's correlation analysis; the Student t test for unpaired samples; and the Mann-Whitney U test, as appropriate. Those determinants with P<.01 were included in a multiple linear regression analysis, also adjusted due to other regulatory factors described (age, thyroid hormones, IGF-1, and cortisol). Cox's proportional hazard model was used to evaluate the univariate association between each variable and the event of the study. Cox's multivariate analysis was used to determine the prognostic value following adjustment for variables with P<.10 in the univariate analysis and confounding factors (age, body mass index [BMI], spironolactone, loop diuretics). The proportional hazards (HR) were expressed with their confidence intervals of 95% (95% CI). The adherence to the assumptions of Cox's proportional hazards model was evaluated using cumulative hazard graphs (Log), time-dependent covariates, and Schoenfeld residuals; being verified with the representation Ln(-Ln St) for each variable after its categorisation. Kaplan-Meier cumulative survival curves were drawn and the log rank values were calculated to evaluate the statistical significance of the differences. A value of P<.05 was considered significant. Statistical analysis was carried out using SPSS 15.0 for Windows computer software (SPSS Inc., Chicago, Illinois).
RESULTS
Sex Hormone Binding Globulin and its Determinants
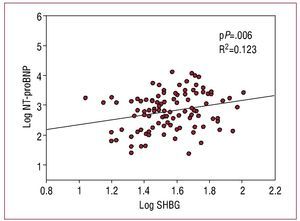
A total of 104 men with HF were included in this study. The clinical characteristics of the population upon inclusion in the study are shown in table 1. The levels of SHBG were 34.5 [IQR, 27-50] nmol/L, and had a significant correlation with the concentration of NT-proBNP (r=0.271, P=.005), the LVEF (r=-0.263, P=.007), and the body mass index (r=-0.200, P=.020). Table 2 shows the levels of the associated hormones; the levels of SHBG demonstrated a positive correlation with testosterone levels, but not with the rest of the hormones. No other significant correlations or differences were observed with the rest of the clinical characteristics and biochemical parameters (P>.1 in all analyses). In a multiple linear regression analysis including total testosterone, NT-proBNP, LVEF and BMI, adjusted for other regulatory factors (age, thyroid hormones, IGF-1, and cortisol), the concentration of NT-proBNP was the only determinant of SHBG levels (P=.006; Figure 1). In contrast with SHBG, total testosterone, and free testosterone did not demonstrate any correlation with the concentration of NT-proBNP, LVEF, or any other clinical characteristic (P>.1).
Figure 1. Scatter diagram of the levels of sex hormone binding globulin versus amino-terminal pro B-type natriuretic peptide.
Sex Hormone-Binding Globulin and Prognostic
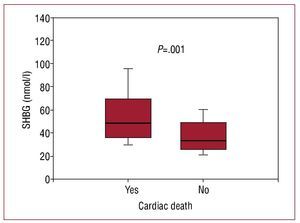
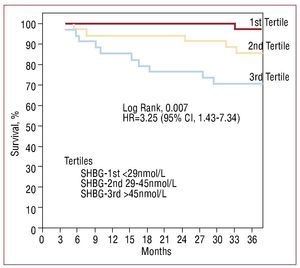
After 3 years of monitoring, 16 patients (15.4%) died, 12 due to refractory HF and 4 due to sudden death. The patients that died showed greater levels of SHBG (nmol/L, 48.5 [36-69.5] vs 33 [25.3-48.7], P=.001; Figure 2). In the Cox univariate analysis (Table 3), the predictors of cardiac death were SHBG, LVEF and NT-proBNP; the rest of the clinical variables and related hormones, including the levels of total testosterone (P=.74) and free testosterone (P=.13), did not have any statistical significance. Following adjustment in a multivariate analysis, the levels of SHBG remained a significant hazard factor (HR=1.056; 95% CI, 1.017-1.096; P=.004). Analysis of SHBG across tertiles (Figure 3) also demonstrated a significant increase in the associated hazard (HR=3.25; 95% CI, 1.43-7.34, P=.004). Mortality after 3 years was at 30% for patients in the upper tertile, 14% in the intermediate tertile, and only 4.3% in the lower tertile (log rank, 0.007).
Figure 2. Box plot of the levels of sex hormone binding globulin according to appearance or non-appearance of cardiac death during the 3-year follow-up period.
Figure 3. Kaplan-Meier survival analysis according to tertiles of sex hormone binding globulin.
DISCUSSION
This study demonstrated for the first time that SHBG, a regulatory protein of anabolic hormonal activity, may play a part in HF. This role is suggested by the findings of the study, in which high levels of SHBG correlate with other parameters of severity of the HF and, in turn, are associated with a worse prognosis in the long term for men with HF.
In recent years, the presence of lower levels of anabolic hormones in patients with HF has been described, in terms of total testosterone, free testosterone, DHEA-S, and IGF-1.3-6 In addition to this, it has been suggested that the presence of anabolic deficiency gives rise to lower survival rates in men with HF.3 The appearance of an imbalance between anabolism and catabolism, in favour of the latter, is associated with increased progression and increased mortality in patients with HF.7 Our study focussed on SHBG, as a key molecule in the action of anabolic steroids, which is regarded as having complex regulation and multiple actions.9-12,19 Due to its binding to sex steroid hormones, it passively determines the biologicallyactive free fraction; therefore, low levels of SHBG give rise to increased biological hormonal activity, and, inversely, high levels give rise to lower anabolic activity. In the cardiovascular system, low levels of SHBG have been associated with hypothyroidism, polycystic ovary syndrome, central obesity, resistance to insulin, dyslipidaemia and an increased cardiovascular risk in men.13,17,18 High levels are associated with anabolic deterioration and a predominance of catabolism, and appear in hypothyroidism, anorexia nervosa, protein-energy malnutrition and some forms of cancer.13-17 However, there is no specific information surrounding its possible role in HF.
Our study demonstrates a relationship between higher levels of SHBG and the presence of HF seventy parameters such as a greater concentration of NT-proBNP and a lower LVEF; in addition to total testosterone, which is its main in vivo regulator. Following multivariate adjustment for known regulatory factors, NT-proBNP appeared as its main determinant in patients with HF. These findings suggest that the levels of SHBG could be influenced by the severity of HF, as has been previously described for DHEA-S; however, testosterone does not show this correlation, neither in our study nor in other previous studies.3,4,6 Regulation of the levels of SHBG is multifactorial and controlled by a balance of stimulant and inhibitory factors, including numerous endocrine, paracrine and growth factors.19 However, regulation of this molecule remains an area for considerable debate and, as our findings suggest, the natriuretic peptide system may also participate.
In our population, high levels of SHBG were associated with an increased risk of cardiac death, even following adjustment for other known risk factors. Given that higher levels of SHBG result in a lower quantity of free hormone, which is biologically active, the increase in SHBG could contribute to a greater anabolic hormone deficiency and/or identify those patients with greater deterioration of this hormonal system. These findings are consistent with those of Jankowska et al, which demonstrated an association between the number of anabolic hormone deficiencies and a worse prognosis in men with HF.3 In addition to this, SHBG also actively regulates the response to steroids in the different tissues, participating in signalling in the cellular membrane and modifying the local effects of steroid hormones.9-11 As a result, the concept of SHBG as a monofunctional protein which binds steroids has changed to a protein with multiple functions, although its actions are still relatively unknown and it could act as an inhibitor to the action of sex steroids in tissues and dependent cells.12 In this respect, if SHBG has a specific effect on the cardiomyocytes in the heart of a patient with heart failure, it has not yet been studied. Our findings suggest that SHBG could play a part in the interaction between the deterioration of anabolic hormones and HF, participating in the pathophysiology of the disorder. However, our study is observational and can only provide a hypothesis in this respect; it is also possible that SHBG is merely an indicator of severity. As a result, the implementation of new studies, which clarify the possible role of SHBG in HF, is necessary.
Levels of SHBG are also an indicator of nutritional state.13-17 Deterioration of the nutritional state results in an increase in SHBG, and high levels of SHBG have been described in patients with anorexia nervosa or protein-energy malnutrition.16,17,22-25 In addition to this, high levels of SHBG result in reduced muscular strength and low bone density; for this reason, a more significant role is attributed to this protein than to the sex steroid hormones in these situations.26-29 Weight loss, cachexia, muscular atrophy and osteoporosis are also findings in the progression of HF, which in turn are associated with a worse prognosis.30 In male patients with coronary heart disease, low levels of SHBG correlate with the presence of central obesity and are associated with a greater risk of cardiovascular events during the follow up period.18,19 In contrast with this population, in patients with HF it is weight loss, not obesity, which results in a worse prognosis.2,30 As a result, the relationship observed in our study between high levels of SHBG and increased mortality would suggest that, in patients with HF, SHBG is also an indicator of malnutrition. However, in this sense the findings are limited given that we only found a weak negative correlation between SHBG and BMI; the lack of a complete nutritional assessment prevents both other associations from being studied and determination of whether this protein may act as an nutritional indicator in patients with HF.
CONCLUSIONS
To conclude, this study demonstrates that in patients with HF, high levels of SHBG correlate with other measurements of severity of the disease and are associated with an increased risk of cardiac death in the follow-up period in the long term. These findings suggest a possible role of SHBG in the pathophysiology of the anabolic deterioration which appears during HF progression, although it is not possible to establish causal relationships due to the fact this is an observational study. Further studies are necessary to define the potential relationships between SHBG and anabolic steroid hormones, nutritional state, and the progression of HF.
ABBREVIATIONS
HF: heart failure
DHEA-S: dehydroepiandrosterone sulfate
IGF-1: insulin-like growth factor-1
NT-proBNP: N-terminal probrain natriuretic peptide
SHBG: sex hormone-binding globulin
SEE ARTICLE ON PAGES 1353-5
This study was funded by a Grant from the Séneca Foundation, the Science and Technology Agency of the Region of Murcia (Project 05822/ PPC/07), and by "REDINSCOR," the National Cooperative Heart Failure Research Network, Ministry of Health and Consumer Affairs, exp. RD06/0003/0013.
Correspondence: Dr. D. A. Pascual Figal.
Unidad de Insuficiencia Cardiaca. Servicio de Cardiología. Hospital Universitario Virgen de la Arrixaca.
Ctra. Madrid-Cartagena, s/n. 30120 Murcia. España.
E-mail: dapascual@servicam.com
Received March 23, 2009.
Accepted for publication July 8, 2009.