Urinary concentrations of amino-terminal pro-B type natriuretic peptide (NT-proBNP) may be prognostically meaningful; however, direct comparison to plasma concentrations of this marker have not been performed in patients with acutely decompensated heart failure (ADHF). The aims of this study were to compare the prognostic value of plasma versus urinary NT-proBNP concentration for the risk stratification of patients with ADHF.
MethodsConsecutive hospitalized patients with ADHF were prospectively studied. Blood and urine samples were simultaneously collected on hospital arrival to determine NT-proBNP concentrations. Clinical follow-up was obtained, and the occurrence of mortality and heart failure hospitalization was registered.
ResultsThe study included 138 patients (median, 74 years [interquartile range, 67-80]; 54% men). During a median follow-up period of 387 days [interquartile range, 161-559], 65 patients (47%) suffered adverse clinical events. Plasma NT-proBNP concentration was higher among patients who presented adverse events (4561 pg/mL [2191-8631] vs 2906 pg/mL [1643-5823]; P=.03), whereas urinary NT-proBNP was similar in both groups (P=.62). After multivariable Cox regression analyses, plasma NT-proBNP concentration was associated with a higher risk of adverse events, whether considered continuously (per 100 pg/mL; hazard ratio [HR]=1.004; 95% confidence interval [CI], 1.001-1.007; P=.003) or categorically (≥3345 pg/mL; HR=2.35; 95% CI, 1.41-3.93; P=.001). In contrast, urinary NT-proBNP concentration was not associated with adverse outcomes.
ConclusionsPlasma NT-proBNP concentration is superior to urinary NT-proBNP concentration for the prediction of adverse clinical outcomes among unselected patients with ADHF.
Keywords
A potential strategy to improve the management of patients with acutely decompensated heart failure (ADHF) involves the use of biomarkers to improve diagnostic and prognostic assessment, the sum total of which may be improvements in therapeutic decision-making. Among cardiac biomarkers, measurement of plasma B-type natriuretic peptide and the amino-terminal fragment of the precursor protein (NT-proBNP) have been shown to be useful in establishing the diagnosis of ADHF and providing prognostic information in patients presenting to urgent care settings with acute dyspnea; in addition, use of these peptides may be useful for triage and in-hospital care of patients with ADHF.1, 2, 3, 4, 5, 6
Recent studies have reported that the urinary measurement of natriuretic peptide concentration may be useful for prediction of adverse outcomes in patients with heart failure (HF).7, 8 However, these studies have predominantly examined selected ambulatory patients with chronic heart failure, and comparisons to plasma measurements were generally not performed. Thus, the applicability of the prognostic usefulness of urinary NT-proBNP concentrations to patients with ADHF remains unclear. The value of a simple urinary screening test for risk is of considerable potential value in ADHF; therefore, in the present study we evaluated the prognostic value of measurements of urinary NT-proBNP, and compared it to that of plasma NT-proBNP concentrations in the risk stratification of a hospitalized population with ADHF.
Methods Study Population and ProtocolThe study population consisted of patients with ADHF from a previously published prospective study.9 From September 2006 to February 2008, we prospectively enrolled 138 consecutive patients admitted with initial diagnoses of ADHF (diagnosed clinically using current guidelines10) to the Department of Cardiology at Virgen de la Arrixaca University Hospital (Murcia, Spain). Blood and urinary samples were simultaneously collected for all patients on arrival at the emergency department.
Baseline clinical characteristics and hospital events were prospectively recorded. Echocardiography was also performed on all patients before hospital discharge. The left ventricular ejection fraction (LVEF) was measured using Simpson's biplane method. All patients received standard management as recommended by contemporary guidelines.10 During the entire hospitalization period, clinical management decisions about each patient were decided by the responsible cardiologist, who was unaware of the patient's NT-proBNP concentrations.
Biochemical AnalysisBlood and urinary samples were simultaneously collected for all patients on arrival at the emergency department. After centrifugation at 1300 rpm and 4°C for 10 minutes, urinary and plasma samples were separated and stored in cryotubes at –80°C until assayed. Before the analysis, the urinary samples were centrifuged twice at 13200 rpm at 4°C for 30 minutes to avoid possible NT-proBNP measurement interferences produced by the precipitation of salts in urine. Plasma and urinary NT-proBNP concentrations were determined by electrochemiluminescence immunoassay using a Modular Analytics E170 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The intra-assay coefficient of variation for NT-proBNP was 1.8% for 221 pg/mL and 3.1% for 4250 pg/mL. To account for possible differences in urinary NT-proBNP concentrations, we also corrected for urinary creatinine concentrations (nanograms per mg urinary creatinine). Estimated glomerular filtration rate (eGFR) was calculated by using the abbreviated version of the Modification of Diet in Renal Disease formula (mL/min/1.73 m,2 186.3×[plasma creatinine] – 1.154×[age] – 0.203) (the correction factor for women was ×0.742).11
Follow-up and Clinical EndpointPatients were clinically followed for a median of 387 days [interquartile range (IQR), 161-559], and a common final date for all was used as the criterion for study termination. Importantly, at the end of follow-up the occurrence of clinical events was registered in all patients. The study endpoints were defined as the combination of mortality and/or HF readmission. Death was ascertained from available medical records and death certificates. If hospital records were ambiguous or unavailable, national death records were consulted. In patients requiring hospitalization, medical records were carefully reviewed to further characterize the cause of hospitalization. The study was approved by the local ethics committee, and informed consent was obtained from each patient at inclusion.
Statistical AnalysisContinuous variables were tested for a normal distribution by the Kolmogorov-Smirnov test. Normally distributed data are presented as the mean±standard deviation and non-normally distributed data as the median [IQR]. Categorical variables are expressed as percentages. Categorized analyses were performed to compare patients who presented adverse clinical events during follow-up and those who did not. Differences in baseline characteristics were compared using t-student or the Mann-Whitney U-test for continuous variables and χ2 test for categorical variables. Relationships between each biomarker and other parameters were assessed by Spearman rank correlation. Multiple linear regression analyses were used to assess the independent effect of clinical variables on urinary NT-proBNP concentrations. Multivariable models were fit through a stepwise selection algorithm using the following variables: age, body mass index, systolic blood pressure, New York Heart Association Functional Classification III-IV, LVEF, atrial fibrillation/flutter, current treatment with aldosterone antagonists on hospital admission, previous history of ST-segment elevation myocardial infarction, eGFR, albumin, troponin T, C-reactive protein and plasma NT-proBNP. Because urinary and plasma NT-proBNP concentrations were not normally distributed, natural logarithmic transformations were used in linear regression analyses; multicollinearity was not detected in any of the models used. The prognosis values of plasma and urinary NT-proBNP were evaluated as continuous and categorical variables (above and below the median concentration). We calculated hazard ratios (HR) derived from the Cox regression analysis to identify predictors of adverse clinical events during follow-up. The independent effect of variables on prognosis was calculated using a Cox multivariable regression analysis, incorporating covariates with P values<0.1 in the univariable analysis. Linearity assumption was tested using Martingale residuals. Log-cumulative hazard plots, time-dependent covariates, and Schoenfeld residuals were used to evaluate adherence to the proportional hazard assumptions of the Cox model. The predictive ability of the final model was quantified using the C-index. The added predictive ability of urinary NT-proBNP over plasma NT-proBNP was tested by calculating the integrated discrimination improvement (IDI), as described by Pencina et al.12. The C-index and the IDI were internally validated by bootstrapping 1000 times using 100% random sampling by replacement. The cumulative incidence of adverse clinical events was estimated according to the Kaplan-Meier method, and the log-rank statistic was used for comparisons. All P values <.05 were accepted as statistically significant. Statistical analysis was performed using SPSS version 15.0 for Windows (SPSS, Inc., Chicago, Illinois, USA).
Results Study PopulationThe clinical characteristics of the study subjects are detailed in Table 1, which describes a relatively typical population of patients with ADHF. The median age is 74 years, and more than half are male (54%). Most subjects had a previous history of hypertension and approximately two thirds of the patients had de novo HF. The median LVEF was 50%; as is typical among patients in contemporary studies of acute HF, 54% had preserved LVEF (LVEF>45%). As expected among subjects with ADHF, the median plasma NT-proBNP concentration was higher than simultaneous urinary NT-proBNP concentration (3345 [1900-7205] pg/mL vs 73 [41-213] pg/mL; P<.001). Patients with reduced LVEF had higher plasma NT-proBNP concentrations than those with preserved LVEF (4298 [2238-10963] pg/mL vs 2933 [1628-5242]; P=.018), whereas urinary NT-proBNP concentrations were similar in patients with either reduced or preserved LVEF (92 [44-225] pg/mL vs 71 [40-195] pg/mL; P=.77). Moreover, patients with moderate-severe kidney dysfunction (eGFR<60mL/min/1.73 m2) had higher plasma and urinary NT-proBNP concentrations than those with normal or near normal kidney function (eGFR=60mL/min/1.73 m2): NT-proBNPplasma, 5897 [2986-12088] pg/mL vs 2252 [1408-4227] pg/mL (P<.001) and NT-proBNPurine, 155 [57-587] pg/mL vs 56 [37-97] pg/mL (P<.001).
Table 1. Baseline Clinical and Biochemical Characteristics of the Entire Cohort
| Variables | Patients (n=138) |
| Age (years) | 74 [67-80] |
| Men | 74 (54) |
| Body mass index | 28 [26-31] |
| Systolic blood pressure (mmHg) | 151±36 |
| Heart rate (beat/min) | 105±31 |
| Left ventricular ejection fraction | 50 [35-60] |
| Prior NYHA functional class III or IV | 44 (32) |
| Chronic heart failure | 86 (62) |
| Coronary heart failure | 48 (35) |
| Diabetes mellitus | 70 (51) |
| Hypertension | 114 (83) |
| Atrial fibrillation/flutter | 86 (63) |
| Previous STEMI | 36 (26) |
| Previous stroke | 22 (16) |
| Anemia | 64 (46) |
| In-hospital inotropic use | 2 (1.4) |
| Hemoglobin (g/dL) | 12.5±2.1 |
| Creatinine (mg/dL) | 1.2 [0.9-1.5] |
| eGFR (mL/min/1.73 m2) | 63±25 |
| Blood urea nitrogen (mg/dL) | 51 [39-72] |
| Albumin (g/dL) | 4.0±0.5 |
| Sodium (mEq/L) | 139±5.6 |
| Uric acid (mg/dL) | 7.7±2.7 |
| C-reactive protein (mg/dL) | 1.2 [0.5-3] |
| Troponin T (ng/mL) | 0.01 [0.01-0.06] |
| Plasma NT-proBNP (pg/mL) | 3345 [1900-7205] |
| Urinary NT-proBNP (pg/mL) | 73 [41-213] |
| Urinary NT-proBNP/Urinary creatinine (pg/mgCr) | 2.7 [1.66-6.49] |
| Current treatment at admission | |
| Beta-blocker | 74 (54) |
| ACE inhibitor/angiotensin II receptor blocker | 72 (55) |
| AA | 47 (34) |
| Loop diuretic | 122 (88) |
AA, aldosterone antagonist; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as median [interquartile range], as mean±SD, or as number (percentage).
Patients on aldosterone antagonists at the time of hospital admission had lower NT-proBNP concentration in urine (43 pg/mL [26-109] vs 72 pg/mL [44-227]; P=.02); however, no other significant differences in urinary NT-proBNP concentration related to clinical conditions or current medication on hospital admission were observed (all P>.05). As shown in Table 2, plasma concentration of NT-proBNP was the main independent predictor of its urinary levels. The adjusted R2 of the model including these variables was 0.55 (P<.001) for log10 urinary NT-proBNP concentration.
Table 2. Correlation Analyses and Independent Determinants of Log10 Urinary N-terminal Pro-B-Type Natriuretic Peptide by Multiple Regression Analysis
| Univariate | Multivariate | |||
| Variables | r | P | β | P |
| Log10 Plasma NT-proBNP | 0.61 | <.001 | 0.52 | <.001 |
| Left ventricular ejection fraction | –0.09 | .282 | 0.17 | .016 |
| Albumin | –0.44 | <.001 | –0.24 | <.001 |
| C-reactive protein | 0.25 | .003 | 0.17 | .009 |
| Current AA use on hospital admission | — | .02 * | –0.15 | .024 |
| Atrial fibrillation/flutter | — | .083 * | –0.16 | .009 |
| Previous STEMI | — | .092 * | 0.14 | .034 |
| Prior NYHA functional class III or IV | — | .308 * | — | .4 |
| Age | 0.22 | .011 | — | .95 |
| Body mass index | –0.06 | .505 | — | .67 |
| Systolic blood pressure | –0.06 | .639 | — | .31 |
| Troponin T | 0.42 | <.001 | — | .25 |
| Estimated GFR | –0.37 | <.001 | — | .13 |
GFR, glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; STEMI, ST-segment elevation myocardial infarction.
* P value for Mann-Whitney U-test.
Over the study period, 65 patients (47%) had adverse clinical events: 33 patients died, and 44 patients were readmitted to hospital owing to HF decompensation. The distribution of characteristics and laboratory parameters by occurrence of adverse clinical events is shown in Table 3. Patients who presented adverse clinical events were older, had a higher prevalence of anemia and chronic heart failure, and needed more frequent in-hospital inotropic support.
Table 3. Baseline Clinical and Laboratory Characteristics of Study Population, According to Mortality and/or Hear Failure Readmission
| No events (n=73) | Events (n=65) | P | |
| Age (years) | 73 [62-79] | 76 [69-82] | .037 |
| Men | 41 (56%) | 33 (51%) | .53 |
| Body mass index | 29 [26-32] | 28 [26-32] | .9 |
| Systolic blood pressure (mmHg) | 156±34 | 148±36 | .21 |
| Heart rate (beat/min) | 100±34 | 99±29 | .98 |
| Left ventricular ejection fraction | 52 [38-60] | 49 [30-60] | .43 |
| Prior NYHA functional class III or IV | 12 (16%) | 32 (49%) | <.001 |
| Chronic heart failure | 38 (52%) | 48 (74%) | .008 |
| Coronary heart failure | 22 (30%) | 26 (40%) | .23 |
| Diabetes mellitus | 32 (44%) | 38 (59%) | .09 |
| Hypertension | 62 (86%) | 52 (80%) | .34 |
| Atrial fibrillation/flutter | 42 (59%) | 44 (68%) | .3 |
| Previous STEMI | 14 (19%) | 22 (34%) | .05 |
| Previous stroke | 10 (14%) | 12 (19%) | .45 |
| Anemia | 27 (27%) | 37 (57%) | .019 |
| In-hospital inotropic use | 6 (8%) | 13 (20%) | .045 |
| Hemoglobin (g/dL) | 13±2 | 12±2 | .01 |
| Creatinine (mg/dL) | 1 [0.8-1.3] | 1.2 [1-1.7] | .01 |
| Estimated GFR (mL/min/1.73 m2) | 69±24 | 56±25 | .003 |
| Blood urea nitrogen (mg/dL) | 47 [36-59] | 58 [47-99] | <.001 |
| Albumin (g/dL) | 4.1±0.4 | 4±0.5 | .18 |
| Sodium (mEq/L) | 138±6 | 138±5 | .64 |
| Uric acid (mg/dL) | 7.5±2.4 | 8±2.9 | .24 |
| C-reactive protein (mg/dL) | 1 [0.6-3] | 1.3 [0.4-3.4] | .93 |
| Troponin T (ng/mL) | 0.01 [0.01-0.04] | 0.02-[0.01-0.06] | .029 |
| Treatment at discharge | |||
| Beta-blockers | 44 (60%) | 30 (53%) | .383 |
| ACE inhibitors/ARB | 64 (88%) | 48 (84%) | .571 |
| AA | 31 (43%) | 16 (28%) | .09 |
| Loop diuretics | 69 (95%) | 53 (93%) | .729 |
AA, aldosterone antagonist; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; GFR, glomerular filtration rate; NYHA, New York Heart Association; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as median [interquartile range], as mean±SD, or as number (%).
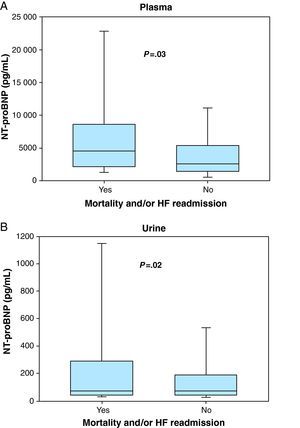
Those with events had higher plasma NT-proBNP concentration (4561 pg/mL [2191-8631] vs 2906 pg/mL [1643-5823]; P=.03) (Figure 1A) but similar urinary NT-proBNP (78 pg/mL [42-294] vs 71 pg/mL [41-189]; P=.62) (Figure 1B) compared to those who did not have events. Serum creatinine, urea nitrogen and troponin T were also higher among patients who presented adverse clinical events; hemoglobin and eGFR were lower among these patients.
Figure 1. Box plots showing the concentrations of plasma (A) and urinary N-terminal pro-B-type natriuretic peptide (B) in patients experiencing mortality and/or heart failure readmission and those who did not have events.HF, heart failure; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
In univariable Cox regression analysis, for each increase of 100 pg/mL in plasma NT-proBNP concentration a higher risk of adverse clinical events was observed, and this association was unchanged after multivariable adjustment (per 100 pg/mL; HR=1.001; 95% CI, 1.004-1.007; P=.003) (Table 4). The C-index in the final model was 0.75 (95% CI, 0.67-0.83) and the bootstrap method demonstrated good internal validation (C-index, 0.75±0.039). In contrast, a 10 pg/mL change in urinary NT-proBNP concentration was not associated with adverse clinical events in the univariable analysis (per 10 pg/mL; HR=1; 95% CI, 0.998-1.001; P=.55) (Table 4). When death or HF readmission were analyzed as separate endpoints, the results were very similar: plasma NT-proBNP was a significant predictor for each event (HR per 100 pg/mL increase in plasma NT-proBNP=1.006; 95% CI, 1.003-1.009; P<.001 for death, and HR=1.003; 95% CI, 1.002-1.004; P=.014 for HF readmission), whereas urinary NT-proBNP was not (P>.3 in both cases).
Table 4. Cox Regression Risk Analyses for Prediction of All Cause Mortality and/or Heart Failure Readmission a
| Univariable | Multivariable b | |||
| HR | P | HR | P | |
| Age | 1.03 (1.01-1.06) | .008 | — | .235 |
| Prior NYHA functional class III or IV | 3.34 (2.03-5.49) | <.001 | 3.58 (2.16-5.93) | <.001 |
| Previous STEMI | 1.81 (1.08-3.04) | .024 | 1.69 (1.01-2.85) | .047 |
| Chronic heart failure | 1.82 (1.05-3.17) | .034 | — | .23 |
| Diabetes mellitus | 1.65 (1.01-2.71) | .049 | — | .08 |
| Anemia | 1.75 (1.07-2.87) | .026 | — | .24 |
| In-hospital inotropic use | 2.17 (1.18-3.99) | .012 | — | .27 |
| Troponin T | 2.61 (1.5-4.55) | .001 | 2.5 (1.36-4.61) | .003 |
| Blood urea nitrogen | 1.007 (1.002-1.012) | .006 | — | .09 |
| Estimated GFR | 2.2 (1.33-3.65) | .002 | — | .09 |
| Plasma NT-proBNP per 100 pg/mL | 1.003 (1.001-1.006) | .01 | 1.004 (1.001-1.007) | .003 |
| Plasma NT-proBNP >3.345 pg/mL | 1.86 (1.13-3.06) | .014 | 2.35 (1.41-3.93) | .001 |
| Urinary NT-proBNP per 10 pg/mL | 1.15 (0.79-1.68) | .55 | ||
| Urinary NT-proBNP >73 pg/mL | 1.2 (0.79-1.96) | .46 | ||
GFR, glomerular filtration rate; HR, hazard ratio; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; STEMI, ST-segment elevation myocardial infarction.
a All variables with a P value <.10 in univariable analysis are shown in the table and were included in the multivariable model.
b Plasma NT-proBNP was tested separately as a quantitative and categorical variable. Multivariable HR andP for other variables shown from quantitative model.
We also assessed the prognostic value of plasma and urinary NT-proBNP concentrations above and below median values. In univariable and multivariable Cox regression analyses, plasma NT-proBNP concentration above the median value (3345 pg/mL) was associated with a higher risk of adverse clinical events (HR=2.35; 95% CI, 1.41-3.93; P=.001) (Table 4). However, urinary NT-proBNP concentration above the median value (73 pg/mL) did not attain statistical significance as a prognostic indicator of events in univariable analysis (HR=1.2; 95% CI, 0.79-1.96; P=.46) (Table 4). Furthermore, when the added predictive value of urinary NT-proBNP over NT-proBNP was tested by calculating IDI, we found that urinary NT-proBNP did not provide additional prognosis information compared to plasma concentration (IDI=0.00786; P=.308, using the bootstrap method).
The normalization of urinary NT-proBNP for urinary creatinine concentrations (pg/mg urinary creatinine) did not improve the results obtained for urinary NT-proBNP. Accordingly, the area under the curve for normalized NT-proBNP concentration in urine was lower: 0.54 (95% CI, 0.45-0.63; P=.049). Univariable Cox regresion analyses also showed that normalized urinary NT-proBNP concentration was not associated with risk of adverse clinical outcomes either as a continuous (per pg/mg; HR=0.997; 95% CI, 0.991-1.004; P=.46) or a categorical variable (>2.7 pg/mg; HR=1.106; 95% CI, 0.68-1.799; P=.68).
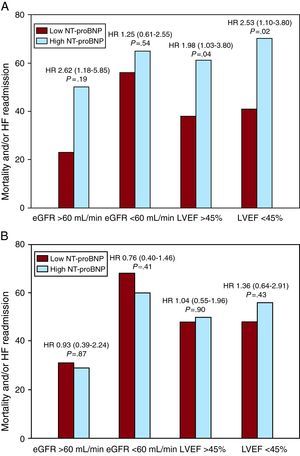
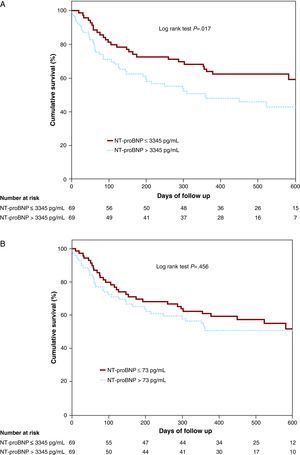
In stratified analyses according to kidney function or LVEF, elevated plasma NT-proBNP was also associated with a higher risk of mortality and/or HF hospitalization in all subgroups, except in patients with moderate-severe kidney dysfunction. By contrast, urinary NT-proBNP did not predict adverse clinical events in any subgroup of patients (Figure 2A and B). Kaplan-Meier survival analyses showed plasma NT-proBNP concentration above the median value was associated with an incremental rate of mortality and/or HF readmission (Figure 3A; log rank test, P=.017), whereas urinary NT-proBNP concentration above the median value was not (Figure 3B; log rank test, P=.465).
Figure 2. Association of plasma (A) and urinary N-terminal pro-B-type natriuretic peptide (B) concentrations with mortality and/or heart failure readmission in patients with acute destabilized heart failure, stratified by estimated glomerular filtration rate and left ventricular ejection fraction. The figure displays the mortality and/or HF readmission risk for participants with plasma and urinary NT-proBNP concentrations above (high) or below (low) the median of 3345 pg/mL and 73 pg/mL, respectively. The hazard ratios (HR) compare high versus low NT-proBNP concentrations between subgroups of participants with eGFR ≥60mL/min/1.73 m2 or <60mL/min/1.73 m2, as well as by LVEF ≥45% or <45%. eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Figure 3. Kaplan-Meier survival curves for mortality and/or heart failure readmission according to plasma (A) or urinary NT-proBNP concentrations (B).NT-proBNP, N-terminal pro-B-type natriuretic peptide.
DiscussionThis study explored the potential prognostic value of urinary NT-proBNP concentration in patients with ADHF, and included a comparison with plasma NT-proBNP in this important clinical scenario. Consistent with prior reports,1, 2, 3, 4 we showed that elevated plasma NT-proBNP concentration was strongly correlated with a higher risk of adverse clinical events in hospitalized patients with ADHF. By contrast, urinary NT-proBNP concentration was not associated with risk of adverse clinical outcomes either as a continuous or a categorical variable in this ADHF cohort.
Plasma natriuretic peptide concentration has been found to be useful as an adjunct to standard clinical evaluation for the diagnosis and prognosis stratification of patients with ADHF.1, 2, 3, 4, 5, 6 As such, the utility of plasma testing for the natriuretic peptides has been recognized and incorporated in consensus documents and guidelines for the diagnosis and management of patients with ADHF.10
On the other hand, little is known about the clinical significance of NT-proBNP concentration in urine at this time. A small number of previous studies found that measurement of natriuretic peptides in urine could be useful for the detection of left ventricular systolic dysfunction, and measurement of natriuretic peptides in the urine may predict adverse clinical events in patients with HF.7, 8, 13, 14 However, not all studies have reported such results. For example, Michielsen et al.15 recently showed that urinary NT-proBNP concentrations present a wide variability within and between individuals, and have a relatively low sensitivity and negative predictive value for the detection of left ventricular systolic dysfunction in patients with ADHF, suggesting that urinary NT-proBNP concentrations should be interpreted with care.
Along these lines, in this study, we showed that urinary NT-proBNP concentration did not predict adverse clinical outcomes in hospitalized patients with ADHF, and these findings remained unchanged after stratifying the population by LVEF or renal function. Of note, our results contrast with those of previous studies that reported an increased risk of adverse cardiovascular events among patients with elevated concentrations of natriuretic peptides in urine, although such studies were typically performed in more stable, ambulatory HF patients7, 8 in contrast to our study of more acutely ill patients; at least in the context of ADHF, our results would suggest urinary natriuretic peptide testing is of less value for prognostication.
As NT-proBNP is a low molecular weight protein (8.5 kDa) that is freely filtered though the glomerulus, we found —as expected— that urinary NT-proBNP increased with increasing plasma NT-proBNP concentration. Indeed, we found plasma NT-proBNP to be the main independent predictor of urinary NT-proBNP concentration. However, we unexpectedly found that urinary NT-proBNP concentrations in our cohort overall were lower than perhaps predicted by the high plasma NT-proBNP concentration observed in our patients with ADHF. In fact, we found that the plasma/urinary ratio in our patients (ratio=46) was at least three times higher than in ambulatory patients with HF, who had relatively comparable urinary NT-proBNP values, but considerably lower plasma values.7, 16 Our results are in contrast to the fact that an increased load of NT-proBNP in the tubular lumen secondary to a higher plasma concentration has been postulated to saturate reabsorptive mechanisms for the peptide, with consequently higher urinary NT-proBNP concentrations. The potential for degradation of NT-proBNP in the urine is highly unlikely, as concentrations of NT-proBNP in our subjects were comparable to other studies; moreover, NT-proBNP is known to be exceptionally stable in urine, making this possibility remote.
The limitations of our study are similar to those of any single-center observational study. The small sample size and relatively small number of patients included in each group also makes it difficult to draw firm conclusions. The validity of our findings in other populations remains to be established. In particular, the value of urinary NT-proBNP concentrations in larger groups of patients with acute HF should be examined. Moreover, no heart transplant recipients were included, so our results could not be extrapolated to this specific population. Since we used the first urine sample upon hospital admission to test urinary NT-proBNP concentration, we could evaluate neither the total amount of urinary NT-proBNP excreted in a full 24-h urine collection nor the NT-proBNP fractional excretion. Another consideration is the lack of standardized methods for urinary NT-proBNP measurement, which would include the use of the same peptide preparation and the same units, references, and cut-off values.17
ConclusionsOur results suggest that, in an unselected ADHF population, plasma NT-proBNP should be preferred over urinary measurement for prognostication. The renal physiology of natriuretic peptide excretion is clearly complex, and merits further investigation.
Conflicts of interestDr. Januzzi reports receiving grant support from Roche Diagnostics, Siemens, and Critical Diagnostics. Dr. Pascual-Figal reports receiving grant support from Roche Diagnostics. No other potential conflicts of interest exist.
Received 27 May 2010
Accepted 3 October 2010
Corresponding author: Departamento de Cardiología, Hospital Universitario Virgen de la Arrixaca, Ctra. Madrid-Cartagena s/n, 30120 Murcia, Spain. sergiosmf13@hotmail.com