Over the last 30years, improvements in antithrombotic therapy and the greater use of mechanical reperfusion have dramatically changed the management of patients with acute coronary syndromes (ACS) and have improved outcomes. Short-term anticoagulation combined with aggressive antiplatelet therapy using acetylsalicylic acid, clopidogrel, and a platelet glycoprotein (GP) IIb/IIIa inhibitor is considered to be the ideal regimen as it allows early coronary intervention and prevents the recurrence of adverse ischemic events. However, the inherent bleeding risk of this intensive pharmacological approach may be responsible for blunting the expected benefit of mechanical reperfusion, as bleeding has been independently associated with early and late mortality.1
The current approach to ACS treatment is therefore focusing on the development of effective strategies with a lower risk of bleeding complications. One of these is the use of bivalirudin, a direct thrombin inhibitor (DTI).
Early clinical trials comparing direct thrombin inhibitors with unfractionated heparinThere is no doubt that DTIs are better pharmacological tools, compared to unfractionated heparin (UFH).2 Their advantages include being independent of antithrombin III levels, better bioavailability, and capacity to inhibit both soluble and clot-bound thrombin, which reduces clot formation and propagation. Furthermore, whereas clot-bound thrombin continues to activate platelets during UFH therapy, DTIs inhibit thrombin-induced platelet activation and thus have indirect antiplatelet activity. Finally, the use of DTIs instead of heparin also avoids the risk of heparin-induced thrombocytopenia, which may occur in 5% to 15% of UFH-treated patients, particularly after prolonged administration.
However, despite these pharmacological advantages, the early studies found that prolonged DTI treatment with little use of percutaneous coronary intervention (PCI) did not lead to any real overall benefit. A meta-analysis3 of 11 studies comparing DTIs with UFH in 35 970 patients with ACS showed equivalent death rates, although those treated with DTIs had a significantly decreased risk of recurrent myocardial infarction (MI) (odds ratio [OR] 0.87, 95% confidence interval [CI] 0.79-0.95; P=.004). In comparison with heparin, the almost irreversible blocker desirudin increased the risk of major bleeding (OR 1.28, 95% CI 1.06-1.55), but this was reduced by the reversible blocker bivalirudin (OR 0.44, 95% CI 0.34-0.56).
Trials of Bivalirudin as Anticoagulant during Percutaneous Coronary Intervention for acute coronary syndromeBivalirudin (Angiomax®, the Medicines Company) is a DTI with a biological half-life of 25min that has been considered a potentially ideal anti-coagulant in cathlabs, as it allows almost on/off use during the procedure. The optimal dose was deduced from a phase II dose-ranging study4 and validated in the REPLACE (Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events)-2 trial.5, 6 These studies showed that the minimum effective dose was a bolus of 0.75mg/kg and an infusion of 1.75mg/kg/h. The ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial used a much lower dose (0.25mg/kg/h) before PCI, which was up-titrated to 1.75mg/kg/h during the procedure.7
The phase III ISAR-REACT (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment) 38 and 3A9 trials compared bivalirudin with UFH alone in patients with stable or biomarker-negative unstable angina. Using a background therapy of acetylsalicylic acid and 600mg of clopidogrel, these studies found that bivalirubin had no advantage over UFH in terms of ischemic outcomes, although bleeding was significantly less when compared with UFH 140 U/kg in ISAR REACT 3, but not when compared with a single bolus dose of UFH 100 U/kg in ISAR REACT 3A.
Taking advantage of the drug's indirect antiplatelet effect and the almost universal concomitant use of acetylsalicylic acid and clopidogrel as background therapy, the following trials compared bivalirudin with heparin plus GPIIbIIIa inhibitors in ACS patients:
– REPLACE-2: 6010 patients with stable angina or ACS without ST-segment elevation (NSTEACS).5, 6
– ACUITY: 13 819 patients with NSTEACS, 7789 (56%) of whom underwent PCI.7, 10
– HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction): 3602 patients with ST-elevation MI (STEMI) who were candidates for primary PCI, 3345 (93%) of whom actually underwent the procedure.11
Taken together, the results of the 3 ACS trials involving a total of 14 784 patients undergoing PCI were similar. In comparison with UFH plus GPIIb/IIIa inhibitors, bivalirudin monotherapy during PCI reduced 30-day major bleeding by 44% (risk ratio [RR] 0.56, 95% CI 0.44-0.72; P<.0001), with a comparable effect on the composite ischemic endpoint of mortality, MI, and revascularization/ischemic target vessel revascularization (RR 1.05, 95% CI 0.95-1.17; P=.31). Thirty-day mortality was equivalent (RR 0.91, 95% CI 0.71-1.17; P=.46), but 1-year mortality was significantly reduced (RR 0.81, 95% C.I. 0.67-0.98; P=.03).6, 10, 12 This reduction was mainly driven by the effect observed in the HORIZONS trial, but there was no statistically significant between-trial heterogeneity (Figure 1).
Figure 1. Mortality benefit at 1 year of bivalirudin compared to unfractionated heparin+glycoprotein IIb/IIIa antagonists in the three acute coronary syndrome trials, with odds ratio and 95% confidence interval. Numbers are taken from references Lincoff et al, 6 Stone et al, 10 and Mehran et al. 12 As far as the Acute Catheterization and Urgent Intervention Triage Strategy trial is concerned, only patients actually undergoing percutaneous coronary intervention have been included. ACUITY, Acute Catheterization and Urgent Intervention Triage Strategy; CI, confidene interval; GP, glycoprotein; GPI: glycoprotein inhibitors; HORIZONS-AMI, Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction; M-H, Mantel-Haenszel; UFH, unfractionated heparin.
Strengths and doubtsOn the basis of the findings of the above studies, using bivalirudin instead of UFH plus a GPIIb/IIIa inhibitor during PCI significantly reduces the risk of periprocedural bleeding and long-term mortality. These effects were most pronounced in the 2 studies of intraprocedural drug use,6, 12 and were achieved at the cost of a slight, nonsignificant increase in 30-day ischemic endpoints (the REPLACE-2 and ACUITY trials) and a significant increase in the incidence of stent thrombosis during the first 24h after primary PCI for STEMI (the HORIZONS trial). In the light of these data, the 2010 European guidelines on myocardial revascularization recommend bivalirudin as an anticoagulant during PCI in patients with ACS (non-ST and ST-elevation) at high risk of bleeding (level of evidence IB).13 However, although bivalirudin is being used in more than 50% of procedures in the United States (SDI Health, October 2010), current registries indicate that its use in Europe is limited to less than 5%.
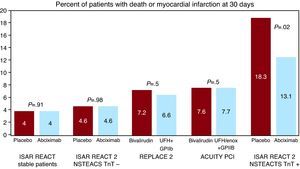
There are various reasons for this rather skeptical approach of European interventionalists to bivalirudin. First of all, it has been shown that the combination of UFH and a GPIIb/IIIa inhibitor is advantageous in patients at higher ischemic risk, but these event rates in the bivalirudin trials were low. Figure 2 compares the 30-day incidence of death and MI in the NSTEACS patients in the REPLACE-2 and ACUITY trials with that observed in the ISAR REACT 114 and 215 trials, which clearly restricted the use of abciximab to higher risk patients (defined as troponin-positive). There is a trend from no effect of GPIIb/IIIa inhibition in the lower risk subsets of the ISAR REACT 1 (patients with stable angina or troponin-negative unstable angina) and ISAR REACT 2 trials (troponin-negative patients with NSTEACS), to a slight but nonsignificant effect in REPLACE-2 and ACUITY (with intermediate event rates in the control group), to a statistically significant and clinically relevant effect in the ISAR REACT 2 troponin-positive patients in whom the ESC guidelines currently restrict the use of GPIIb/IIIa inhibitors. A similar trend in the efficacy of GPIIb/IIIa blockers has been shown in the case of primary PCI for STEMI: a meta-regression analysis of all primary PCI trials investigating the effect of GPIIb/IIIa inhibition found a close correlation with benefit in terms of mortality in comparison with rates observed in the control groups of the individual trials, with the HORIZONS trial at the lower end of the risk scale.16
Figure 2. Impact of GPIIb/IIIa inhibitors on top of acetylsalicylic acid and clopidogrel depends on patients’ baseline risk (studies ISAR REACT 14 , ISAR REACT 2 15 , REPLACE 2 5 , ACUITY PCI 10 ). ACUITY, Acute Catheterization and Urgent Intervention Triage Strategy; GP, glycoprotein; ISAR-REACT, Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment; NSTEACTS, non-ST elevation acute coronary syndrome; PCI, percutaneous coronary intervention; TnT, troponin T; UFH, unfractionated heparin.
A second criticism is the excessive dose of UFH (or level of anticoagulation) in the control groups of most bivalirudin trials (Table 1). This was specifically investigated in the ISAR REACT 3A9 study of PCI in stable patients, which showed that reducing the preprocedural UFH bolus from 140 U/kg (as it was in ISAR REACT 38) to 100 U/kg lowers the risk of procedural bleeding to a level that is nonsignificantly higher than that obtained using bivalirudin, with similar (or slightly better) ischemic outcomes. Where bivalirudin was compared with UFH plus a GPIIb/IIIa inhibitor in ACS patients, the peak procedural ACT in the combination groups of both REPLACE-25 and HORIZONS17 was 310s, as against the 200-250s currently recommended.18 These higher levels were observed in HORIZONS despite the detailed protocol instructions to use a UFH bolus of 60 U/kg and subsequent nomogram-guided dose adjustments to keep ACT between 200 s and 250s11 possibly because 65% of the patients had received UFH before randomization.
Table 1. Heparin Dosage and Peak Activated Clotting Time Levels in Unfractionated Heparin Groups of Bivalirudin Studies.
| Study | Heparin dosage | ACT level | My practice |
| ISAR REACT 3 (Kastrati et al 8 ) | 140 U/kg | 70 U/kg | |
| ISAR REACT 3A (Schulz et al 9 ) | 100 U/Kg | 70 U/kg | |
| REPLACE-2 (Lincoff et al 5 ) | 320 s with GPIIb/IIIa | 200-250 s | |
| HORIZONS (Wohrle et al 17 ) | 310 s with GPIIb/IIIa | 200-250 s |
ACT: activated clotting time; GP, gycoprotein; UFH, unfractionated heparin.
One further point that may reduce the appeal of bivalirudin among European interventionalists is the current shift from femoral to radial catheterization, which has been associated with significantly less major bleeding (absolute risk reduction 1.8%, 95% CI 1.0%-2.5%; P=.001; number needed to treat [NNT] to prevent 1 major bleeding: 56).19 Whereas 95% of the patients were treated femorally in the HORIZONS trial, this proportion is now much lower, particularly in Europe.
Primary percutaneous coronary intervention and acute stent thrombosisDespite the limitations regarding the study population and excessive anticoagulation in the control group, the results of the HORIZONS trial remain striking because the reduction in all-cause mortality after 1 year (persisting for up to 3years) was both statistically and clinically significant (4.8% vs 3.4%; HR 0.69, 95% CI 0.50-0.97; P=.029),12 and was attributed to a 40% reduction in the incidence of major periprocedural bleeding (8.3% vs 4.9%; HR 0.60, 95% CI 0.46-0.77; P<.0001). This benefit was observed despite an increased incidence of stent thrombosis (ST) in the bivalirudin group during the first 24h (1.5% vs 0.3%; HR 5.93, 95% CI 2.07-17.04; P=.0002).11
There may be 2 reasons why bleeding had a greater impact on mortality than on ST. The first is that most of the cases of stent thrombosis in the bivalirudin group occurred during the first 24h (21/54 vs 4/40 in the GPIIb/IIIa+UFH group) when the patient was still hospitalized and a prompt intervention could be performed. The second is that the absolute incidence of major bleeding was higher than that of ST: although the associated HR of death was higher for ST (10.62, 95% CI 3.96-28.48 vs 6.22, 95% CI 3.33-11.60), the fact that more patients experienced major bleeding (195 patients, 18 deaths) than ST (57 cases, 5 deaths) during the 30days following the procedure means that 8.3% of the 54 deaths observed in the study as a whole can be attributed to ST and 28% to bleeding events. It is tempting to conclude that the interventionalists who drive the use of bivalirudin are much more worried about ST than about bleeding, which is more likely to be addressed by using the radial approach to catheterization or closure devices in the case of the femoral approach.
One other conclusion with regard to the HORIZONS ST data is that the combination of acetylsalicylic acid, clopidogrel, and bivalirudin is suboptimal for the prevention of acute ST, the occurrence of which can be dramatically reduced by GPIIb/IIIa inhibitors and prasugrel20 (ie, drugs that induce more prompt and powerful platelet inhibition than clopidogrel). Testing the combination of acetylsalicylic acid and prasugrel upon first medical contact, followed by the procedural use of bivalirudin, should be considered one of the next steps for randomized clinical trials of primary PCI.
Tailoring the use of bivalirudin in patients at high risk of bleedingBivalirudin allows early PCI in ACS patients, with a lower risk of bleeding than other pharmacological strategies, which is why the current revascularization guidelines10 recommend its use in patients at high risk of bleeding. However, only the REPLACE-2 trial found a preferential benefit in terms of 1-year mortality in patients at higher bleeding risk, such as the elderly, diabetics, women, and patients with renal dysfunction.5, 6 In the HORIZONS trial, although bivalirudin reduced bleeding events among older patients (the NNT to prevent 1 event was 16 in the patients aged > 75years and 40 in those aged<75years), the extent of the mortality benefit was no greater.21 Similarly, no specific advantage in terms of 1-year mortality was observed among the patients at higher risk of bleeding in the ACUITY trial.10 The guideline recommendation of the tailored use of bivalirudin in patients at higher risk of bleeding is therefore logical, but only weakly supported by the available data.
ConclusionsIn comparison with UFH, bivalirudin has pharmacokinetic and pharmacodynamic advantages that make it particularly attractive during PCI in ACS. However, its much higher cost requires consideration of its clinical benefit. The results of the ISAR REACT-3 and -3A studies exclude a clinical advantage in stable patients, particularly if European UFH doses are used. It seems to be better to use bivalirudin rather than UFH plus GPIIb/IIIa inhibitors during the procedure in the case of ACS patients at high risk of bleeding. However, clinical trials have mainly compared it with GPIIb/IIIa blockers in low-risk patients, in whom the use of GPIIb/IIa inhibitors is not recommended by the current guidelines. A fair comparison with GPIIb/IIIa inhibitors should be restricted to subsets of patients at high ischemic risk together with lower levels of anticoagulation than those used in the bivalirudin trials. The increased risk of acute ST after primary PCI for STEMI needs to be adequately addressed: a strategy based on an early loading dose of prasugrel (or maybe ticagrelor in the near future), which can block platelets within 1h to 2h of administration, and the procedural use of bivalirudin should be tested in clinical trials.
Conflicts of interestNone declared.
Corresponding author: Prima Divisione di Cardiologia, Ospedale Niguarda Ca’ Granda, Piazza Ospedale Maggiore 3, 20162 Milano, Italy. Stefano.savonitto@fastwebnet.it