Cardiac resynchronization therapy with a defibrillator prolongs survival and improves quality of life in advanced heart failure. Traditionally, patients with ejection fraction > 35 estimated by echocardiography have been excluded. We assessed the prognostic impact of this therapy in a group of patients with severely depressed systolic function as assessed by echocardiography but with an ejection fraction > 35% as assessed by cardiac magnetic resonance.
MethodsWe analyzed consecutive patients admitted for decompensated heart failure between 2004 and 2011. The patients were in functional class II-IV, with a QRS ≥ to 120 ms, ejection fraction ≤ 35% estimated by echocardiography, and a cardiac magnetic resonance study. We included all patients (n=103) who underwent device implantation for primary prevention. Ventricular arrhythmia, all-cause mortality and readmission for heart failure were considered major cardiac events. The patients were divided into 2 groups according to systolic function assessed by magnetic resonance.
ResultsThe 2 groups showed similar improvements in functional class and ejection fraction at 6 months. We found a nonsignificant trend toward a higher risk of all-cause mortality in patients with systolic function ≤ 35% at long-term follow-up. The presence of a pattern of necrosis identified patients with a worse prognosis for ventricular arrhythmias and mortality in both groups.
ConclusionsWe conclude that cardiac resynchronization therapy with a defibrillator leads to a similar clinical benefit in patients with an ejection fraction ≤ 35% or > 35% estimated by cardiac magnetic resonance. Analysis of the pattern of late gadolinium enhancement provides additional information on arrhythmic risk and long-term prognosis.
Keywords
Previous clinical trials have shown that, in advanced heart failure (HF), cardiac resynchronization therapy (CRT), with or without an implantable cardioverter defibrillator (ICD), increases survival and improves quality of life compared with optimal medical therapy in asymptomatic patients in sinus rhythm with a QRS of 120ms or more and a left ventricular ejection fraction (LVEF) of 35% or less assessed by echocardiography.1–4 Other studies have demonstrated the beneficial role of defibrillators in primary prevention in patients who meet these same criteria.5,6
Traditionally, these trials have excluded patients with moderate systolic dysfunction. However, the technical limitations and interobserver variability of echocardiography in the accurate determination of LVEF are well known, and it continues to be an imprecise method.7,8 Cardiac magnetic resonance (MR), on the other hand, is considered to be a more accurate and reproducible technique for the evaluation of overall and regional myocardial function.9 Moreover, it enables analysis of necrotic areas through detection of late gadolinium enhancement (LGE),10 and thus, at the present time, is considered the gold standard for identifying and quantifying the extent of necrosis.11 The relationship between the presence of LGE and the development of ventricular arrhythmias is firmly established,12 and mortality studies have demonstrated that MR has a prognostic ability independent of LVEF.13
Recent studies have evaluated the beneficial effect of CRT for patients with an LVEF > 35%, whether based on echocardiograms analyzed subsequently in experienced core laboratories14 or on estimations in comparative studies involving MR images.15 These reports have found similar benefits in patients with an LVEF > 35% compared with patients with severe dysfunction, both in clinical improvement and in the incidence of major cardiovascular events. Despite the increasing evidence, no studies have previously evaluated the risk of arrhythmias in patients with moderate dysfunction who have undergone CRT implantation with a defibrillator (CRT-D).
Thus, we decided to analyze the prognostic impact of CRT-D in a group of patients with severe dysfunction estimated by echocardiography but reclassified as having an LVEF > 35% on MR study, including the detection of ventricular arrhythmias and their predisposing factors.
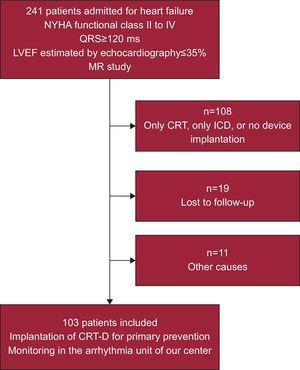
METHODSStudy PopulationWe studied 241 consecutive patients in New York Heart Association functional class II-IV, with QRS ≥ 120ms and LVEF ≤ 35% assessed by echocardiography. All patients had been admitted to our center with a diagnosis of decompensated HF between January 2004 and December 2011, and had undergone MR as part of a local protocol for advanced HF. In the final sample, we included all patients (n=103; mean age, 65 [11] years; 69% were men) who had undergone CRT-D implantation for primary prevention and were followed up regularly in the arrhythmia unit. With the exception of the conditions described, there were no clinical differences between excluded patients and the final sample (Fig. 1). The study received the approval of the ethics committee of the center and all patients signed an informed consent form prior to undergoing MR.
Flow chart showing the patients included in the study. CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy with defibrillator; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; MR, magnetic resonance; NYHA, New York Heart Association.
Two experienced cardiologists performed a 2-dimensional transthoracic echocardiogram in all patients prior to device implantation. The study included measurement of left ventricular diameters and LVEF calculation using the modified Simpson method. The examination was repeated 6 months after implantation to evaluate changes in LVEF and in ventricular dimensions. The studies were performed with the models Philips i33® and Philips Sonos 7500® (Philips Healthcare; United States).
Cardiac Magnetic ResonanceThis study was carried out according to a protocol, within a period no longer than 30 days after the initial echocardiogram, using a 1.5 T system (Magnetom Sonata®, Siemens; Germany). The protocol included determination of LVEF and left ventricular end-diastolic and end-systolic volumes indexed to body surface area, by means of manual tracing of the endocardial and epicardial borders in short-axis steady-state free precession cine sequences from the mitral plane to the ventricular apex. Necrosis was considered to be present when the LGE pattern was subendocardial or transmural, and myocardial fibrosis was identified when the pattern was intramyocardial, focal, or linear, following intravenous administration of gadobenate dimeglumine (Multihance®; Bracco Spa; Italy) at 0.15 mmol/kg body weight according to the standard protocol. Image analysis was carried out in a specific work station (Argus, Siemens; Germany).
Implantation and Follow-up of the Cardiac Resynchronization Therapy Devices With DefibrillatorThe indication for device implantation was based on clinical guidelines.16–18 The therapies were programmed by 2 expert electrophysiologists according to the standard protocol.19 In general, 3 zones of tachyarrhythmia detection were established: a ventricular fibrillation zone, for which defibrillation therapy was programmed; a rapid ventricular tachycardia zone, with programming of antitachycardia pacing, and a slow ventricular tachycardia zone, generally programmed in monitoring mode. The follow-up of the arrhythmic events was carried out periodically every 3 to 6 months in the arrhythmia unit of our center. At each visit, the patient was evaluated clinically, the medical therapy was optimized, and the device was interrogated to record any antiarrhythmic event. For the outcomes analysis, we considered the therapies corresponding to the defibrillator: antitachycardia pacing upon detection of ventricular tachycardia and appropriate shock upon detection of ventricular tachycardia or ventricular fibrillation.
Patient Follow-upThe incidents considered to be major cardiovascular events during follow-up were hospital admission due to HF and all-cause mortality, including sudden death. Ventricular arrhythmias requiring device activation were also included in the analysis as adverse arrhythmic events.
Statistical AnalysisThe continuous variables are expressed as the mean (standard deviation) and the categorical variables as absolute values and percentages. The patients in the sample were divided into 2 groups according to their MR-LVEF (≤ 35% or > 35%), and the baseline characteristics were compared using Student's t test for independent samples or the chi-square test, respectively. Changes in the continuous variables during follow-up with respect to baseline values were analyzed using Student's t test for paired samples. The prognostic predictors of the incidence of major cardiovascular events were determined in a univariate analysis. All statistically significant variables were subsequently included in a multivariate Cox regression analysis and proportional hazards were expressed as hazard ratio (HR) with its 95% confidence interval (95%CI). Survival curves were constructed according to the Kaplan-Meier method, and groups were compared using the log rank test. In the analysis of the results, a P value < .05 was considered to indicate statistical significance. The statistical study was performed using version 17.0 of the SPSS software package (SPSS Inc.; United States).
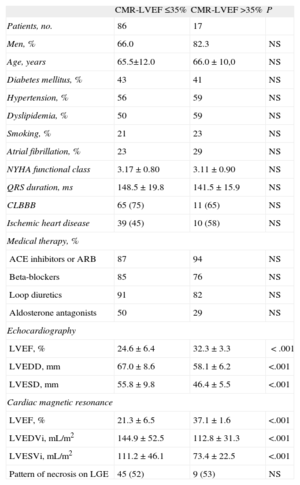
RESULTSThe baseline characteristics of the sample are shown in Table 1. Patients with MR-LVEF > 35% had smaller ventricular diameters and volumes, with a similar distribution of cardiovascular risk factors and medical therapy. The 2 groups were homogeneous with respect to the prevalence of atrial fibrillation, underlying ischemic heart disease, and pattern of necrosis estimated by MR, as well as electrocardiographic findings prior to implantation in terms of both the QRS width and the prevalence of left bundle branch block.
Baseline Characteristics of the Patients According to Left Ventricular Ejection Fraction Estimated by Cardiac Magnetic Resonance
| CMR-LVEF ≤35% | CMR-LVEF >35% | P | |
| Patients, no. | 86 | 17 | |
| Men, % | 66.0 | 82.3 | NS |
| Age, years | 65.5±12.0 | 66.0±10,0 | NS |
| Diabetes mellitus, % | 43 | 41 | NS |
| Hypertension, % | 56 | 59 | NS |
| Dyslipidemia, % | 50 | 59 | NS |
| Smoking, % | 21 | 23 | NS |
| Atrial fibrillation, % | 23 | 29 | NS |
| NYHA functional class | 3.17±0.80 | 3.11±0.90 | NS |
| QRS duration, ms | 148.5±19.8 | 141.5±15.9 | NS |
| CLBBB | 65 (75) | 11 (65) | NS |
| Ischemic heart disease | 39 (45) | 10 (58) | NS |
| Medical therapy, % | |||
| ACE inhibitors or ARB | 87 | 94 | NS |
| Beta-blockers | 85 | 76 | NS |
| Loop diuretics | 91 | 82 | NS |
| Aldosterone antagonists | 50 | 29 | NS |
| Echocardiography | |||
| LVEF, % | 24.6±6.4 | 32.3±3.3 | <.001 |
| LVEDD, mm | 67.0±8.6 | 58.1±6.2 | <.001 |
| LVESD, mm | 55.8±9.8 | 46.4±5.5 | <.001 |
| Cardiac magnetic resonance | |||
| LVEF, % | 21.3±6.5 | 37.1±1.6 | <.001 |
| LVEDVi, mL/m2 | 144.9±52.5 | 112.8±31.3 | <.001 |
| LVESVi, mL/m2 | 111.2±46.1 | 73.4±22.5 | <.001 |
| Pattern of necrosis on LGE | 45 (52) | 9 (53) | NS |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CLBBB, complete left bundle branch block; CMR-LVEF, left ventricular ejection fraction estimated by cardiac magnetic resonance; LGE, late gadolinium enhancement; LVEDD, left ventricular end-diastolic diameter in parasternal long-axis view; LVEDVi, left ventricular end-diastolic volume indexed to the body surface area; LVESD, left ventricular end-systolic diameter in parasternal long-axis view; LVESVi, left ventricular end-systolic volume indexed to the body surface area; NS, not significant; NYHA, New York Heart Association.
Statistically significant differences, P≤.05.
Unless otherwise indicated, data are expressed as no. (%) or mean±standard deviation.
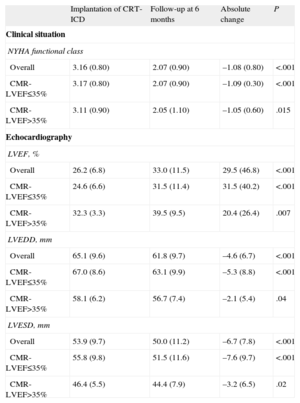
When we analyzed the clinical effects of CRT, we found similar benefits in functional class in both groups in the follow-up at 6 months (−1.05 [0.6] in MR-LVEF > 35% [P=.01] vs –1.09 [0.3] in MR-LVEF ≤ 35% [P<.001]). Likewise, we observed no significant differences between the 2 groups with respect to either improvement in systolic function (proportional improvement in LVEF, 20.4% [26.4%] vs 31.5% [40.2%]; Student's t test for independent samples; P=.09) or ventricular diameters (percent change in left ventricular end-diastolic diameter, −2.1% [5.4%] vs −5.3% [8.8%] [P=.37]; percent change in left ventricular end-systolic diameter, −3.2% [6.5%] vs −7.6% [9.7%] [P=.39]) (Table 2).
Clinical and Echocardiographic Follow-up After Cardiac Resynchronization Therapy in the 2 Groups of the Sample
| Implantation of CRT-ICD | Follow-up at 6 months | Absolute change | P | |
| Clinical situation | ||||
| NYHA functional class | ||||
| Overall | 3.16 (0.80) | 2.07 (0.90) | –1.08 (0.80) | <.001 |
| CMR-LVEF≤35% | 3.17 (0.80) | 2.07 (0.90) | –1.09 (0.30) | <.001 |
| CMR-LVEF>35% | 3.11 (0.90) | 2.05 (1.10) | –1.05 (0.60) | .015 |
| Echocardiography | ||||
| LVEF, % | ||||
| Overall | 26.2 (6.8) | 33.0 (11.5) | 29.5 (46.8) | <.001 |
| CMR-LVEF≤35% | 24.6 (6.6) | 31.5 (11.4) | 31.5 (40.2) | <.001 |
| CMR-LVEF>35% | 32.3 (3.3) | 39.5 (9.5) | 20.4 (26.4) | .007 |
| LVEDD, mm | ||||
| Overall | 65.1 (9.6) | 61.8 (9.7) | –4.6 (6.7) | <.001 |
| CMR-LVEF≤35% | 67.0 (8.6) | 63.1 (9.9) | –5.3 (8.8) | <.001 |
| CMR-LVEF>35% | 58.1 (6.2) | 56.7 (7.4) | –2.1 (5.4) | .04 |
| LVESD, mm | ||||
| Overall | 53.9 (9.7) | 50.0 (11.2) | –6.7 (7.8) | <.001 |
| CMR-LVEF≤35% | 55.8 (9.8) | 51.5 (11.6) | –7.6 (9.7) | <.001 |
| CMR-LVEF>35% | 46.4 (5.5) | 44.4 (7.9) | –3.2 (6.5) | .02 |
CMR-LVEF, left ventricular ejection fraction estimated by cardiac magnetic resonance; CRT-ICD, cardiac resynchronization therapy with implantable cardioverter defibrillator; LVEDD, left ventricular end-diastolic diameter in parasternal long-axis view; LVESD, left ventricular end-systolic diameter in parasternal long-axis view; NYHA, New York Heart Association.
Comparative analysis using Student's t test.
Data are expressed as mean (standard deviation).
In contrast, when we evaluated major cardiovascular events over the long term (mean follow-up, 60 [1-110] months), we observed a nonsignificant trend toward a higher overall mortality rate in patients with MR-LVEF ≤ 35% (log rank test, P=.109), with a similar risk with respect to the incidence of ventricular arrhythmias or hospital admission due to HF in the 2 groups (Fig. 2).
Kaplan-Meier curves based on the left ventricular ejection fraction estimated by cardiac magnetic resonance: cumulative survival corresponding to ventricular arrhythmias, readmission due to heart failure, and all-cause mortality. CMR-LVEF, left ventricular ejection fraction according to cardiac magnetic resonance; CRT-D, cardiac resynchronization therapy with defibrillator.
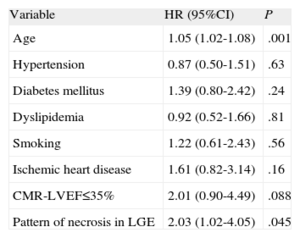
On the basis of the additional information provided by MR, we decided to study whether the LGE pattern had a long-term impact on prognosis and whether it was independent of the severity of systolic dysfunction, as reported in previous studies. For this purpose, we carried out a multivariable analysis using Cox regression analysis in which, in addition to age and the pattern of necrosis as the only factors showing statistical significance in the univariable analysis, the variables included conventional risk factors, a history of ischemic heart disease, and severe systolic dysfunction as established by MR (Table 3). We found that both age and the pattern of necrosis estimated by MR behaved as independent risk factors in the prediction of adverse cardiovascular events in these patients (HR=1.05 [95%CI, 1.02-1.08]; P=.001; and HR=2.03 [95%CI, 1.02-4.05]; P=.045, respectively).
Multivariate Analysis Using Cox Regression: Estimate of the Proportional Risk for Ventricular Arrhythmia, Readmission Due to Heart Failure, and All-cause Mortality During Long-term Follow-up
| Variable | HR (95%CI) | P |
| Age | 1.05 (1.02-1.08) | .001 |
| Hypertension | 0.87 (0.50-1.51) | .63 |
| Diabetes mellitus | 1.39 (0.80-2.42) | .24 |
| Dyslipidemia | 0.92 (0.52-1.66) | .81 |
| Smoking | 1.22 (0.61-2.43) | .56 |
| Ischemic heart disease | 1.61 (0.82-3.14) | .16 |
| CMR-LVEF≤35% | 2.01 (0.90-4.49) | .088 |
| Pattern of necrosis in LGE | 2.03 (1.02-4.05) | .045 |
95%CI, 95% confidence interval; CMR-LVEF, left ventricular ejection fraction estimated by cardiac magnetic resonance; HR, hazard ratio; LGE, late gadolinium enhancement.
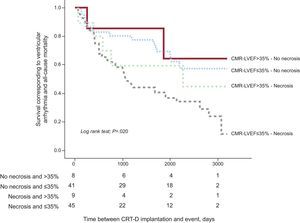
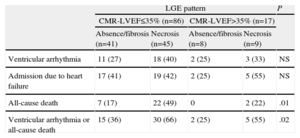
With these findings, we constructed a Kaplan-Meier survival curve, dividing the sample into 4 groups depending on the MR-LVEF and the presence of necrosis according to LGE detected by MR (Table 4). We observed that the presence of a pattern of necrosis enabled us to identify the patients with the highest risk in the combination of ventricular arrhythmias and all-cause mortality during follow-up in both groups (MR-LVEF > 35% and ≤ 35%). Moreover, the combination of MR-LVEF > 35% and the absence of necrosis in LGE images was associated with a better prognosis (Fig. 3).
Major Cardiovascular Events During Follow-up. Distribution According to Late Gadolinium Enhancement and Systolic Function Estimated by Cardiac Magnetic Resonance
| LGE pattern | P | ||||
| CMR-LVEF≤35% (n=86) | CMR-LVEF>35% (n=17) | ||||
| Absence/fibrosis (n=41) | Necrosis (n=45) | Absence/fibrosis (n=8) | Necrosis (n=9) | ||
| Ventricular arrhythmia | 11 (27) | 18 (40) | 2 (25) | 3 (33) | NS |
| Admission due to heart failure | 17 (41) | 19 (42) | 2 (25) | 5 (55) | NS |
| All-cause death | 7 (17) | 22 (49) | 0 | 2 (22) | .01 |
| Ventricular arrhythmia or all-cause death | 15 (36) | 30 (66) | 2 (25) | 5 (55) | .02 |
CMR-LVEF, left ventricular ejection fraction estimated by cardiac magnetic resonance; LGE, late gadolinium enhancement; NS, not significant.
The data are expressed as no. (%).
Kaplan-Meier curve based on the left ventricular ejection fraction and the pattern of necrosis assessed by cardiac magnetic resonance: survival corresponding to cardiovascular death and ventricular arrhythmias. CMR-LVEF, left ventricular ejection fraction according to cardiac magnetic resonance; CRT-D, cardiac resynchronization therapy with defibrillator.
This 8-year observational study included patients who were admitted to our center for acute HF decompensation with severe systolic dysfunction as assessed by the initial echocardiographic study, who underwent implantation of a CRT-D device in accordance with the indications recommended in guidelines.
The retrospective data analysis enabled us to divide the sample into 2 groups; 16% of the patients had an LVEF > 35%, as assessed by MR prior to device implantation. We observed that the 2 groups benefited comparably following implantation of the CRT-D, with improvements in both functional class and in systolic function and ventricular volumes. This significantly favorable outcome in both groups agrees with the findings of previous studies,13,14 and similar benefits were observed in the clinical features and echocardiographic variables in both the patients with severe dysfunction assessed by to the 2 imaging techniques and in those reclassified as having moderate dysfunction following analysis of the MR images.
In addition, on evaluating the major cardiovascular events during long-term follow-up, we found a comparable risk in the incidence of ventricular arrhythmias and readmissions due to HF in both groups, with a nonsignificant trend toward a higher all-cause mortality rate among patients with a MR-LVEF ≤ 35%. These results are also in line with those reported by Foley et al.,14 who observed a trend toward a greater risk of all-cause mortality or a combination of death and hospital admission due to HF among patients with an MR-LVEF ≤ 35%, which was not statistically significant. This was probably because of the small sample size, as is the case in our study.
Importantly, the cut-off for LVEF of ≤ 35% accepted by clinical practice guidelines is not the result of prospective multicenter studies; rather, it was taken from the inclusion criteria used in the major clinical trials involving HF and CRT.1–6 Thus, according to the information published to date and despite demonstration of the association between the degree of systolic dysfunction and the risk of cardiovascular events, perhaps the numerical value of LVEF ≤ 35% on echocardiography should not be accepted as the threshold for an adverse prognosis in these patients. Moreover, echocardiography continues have a number of limitations, such as dependence on the operator, the variable acoustic window, and inadequate visualization of the endocardial borders, in addition to dependence on geometric assumptions. Likewise, LVEF is by no means a constant parameter and can vary in different clinical situations. Thus, the use of specific numerical values to determine the degree of left ventricular dysfunction should not be an exclusion criterion for the implantation of CRT-D devices. In this respect, the use of the combined information provided by echocardiography and MR improves individual patient management of each patient and would probably come closer to current evidence.
Perhaps the most notable finding of this study is the added value provided by the pattern of necrosis on MR with regard to an LVEF ≤ 35% as a strict echocardiographic criterion in the prediction of adverse events, especially concerning the risk of arrhythmia and of all-cause mortality. The prognostic role of LGE showing an ischemic pattern in the risk stratification of patients with indication for an ICD is well known, and this technique has been shown to be able to predict ventricular arrhythmias independently of LVEF.20 Moreover, the prognostic importance of the size of the myocardial scar has recently been proposed, relating a greater extent of the transmural scar, and not only the qualitative presence of LGE, to an increased risk of adverse events in these patients.10,21
On the basis of this same hypothesis, the DETERMINE trial was designed in 2009.22 This prospective, multicenter, randomized trial included patients with coronary artery disease and moderate systolic dysfunction (LVEF > 35%) who underwent cardiac MR to determine the size of the infarction. The objective was to demonstrate that patients with necrotic scarring affecting > 10% of the total myocardial mass, randomized to ICD plus optimal medical therapy, would have reduced mortality compared with those receiving only medical therapy. Unfortunately, the trial was discontinued prematurely because of the small number of patients enrolled, although the role of MR in this clinical context continues to increase.
Thus, as indicated by the current recommendations,16,18 MR prior to implantation is useful in the evaluation of cardiac function and adds detailed information on the extent of the necrotic scar and the viable myocardium, an aspect that is of vital importance in the placement of the electrodes of the device. As indicated by our results, MR would also allow optimization of prognostic evaluation in patients with an indication for CRT-D because of the added value of the pattern of necrosis in the LGE images over the quantitative determination of LVEF by echocardiography.
Study LimitationsWe consider as limitations to the study both the reduced sample size, with only 17 patients in the group with moderate dysfunction on MR, and the fact that it was carried out in a single center and was based on a retrospective analysis of the data. However, given that the objective of the study was to substantiate the idea that the numerical value assigned to LVEF estimated by echocardiography should not be a restrictive criterion in the indication for implantation of CRT-D devices, as proposed in the discussion, the authors consider that this report fulfills its aim to generate new hypotheses. Thus, in the future, further studies will be necessary to confirm the concept indicated by these results.
In addition, although we used the register of appropriate therapies provided by the ICD in the results as a criterion for the indirect evaluation of its potential benefit in patient prognosis, such as the reduction of the risk of sudden death, this register is not equivalent to prevention of cardiac death in all patients since it partly depends on device programming and the type of ventricular arrhythmia. Moreover, prevention of sudden death does not always imply a significant increase in life expectancy, since some patients subsequently die of HF or other causes.23
CONCLUSIONSThe clinical benefits of implantation of a CRT-D for primary prevention in patients with severe dysfunction assessed by echocardiography were similar in both the group of patients with MR-LVEF ≤ 35% and in those reclassified as having moderate dysfunction. The analysis of the LGE pattern in the MR performed prior to device implantation provided additional information in the evaluation of arrhythmic risk and the long-term prognosis in these patients.
FUNDINGThis study was carried out with the financial support of projects from the Spanish Health Care Research Fund (PI 04/2579, PI 07/1039, PI 10/01112), Instituto de Salud Carlos III, Ministry of Health and Consumer Affairs, and the I3 Program (2007, 2011, and 2012) of the Instituto de Salud Carlos III and the Generalitat Valenciana, Spain, granted to Dr. Francisco Ridocci-Soriano.
CONFLICTS OF INTERESTNone declared.