Keywords
INTRODUCTION
Kearns-Sayre syndrome (KSS) is a mitochondrial cytopathy that was first described in 1958.1 The diagnostic criteria include pigmentary retinopathy, chronic progressive external ophthalmoplegia (CPEO) and one or more of the following: cardiac conduction defects, cerebellar ataxia, or cerebrospinal fluid protein concentration >1.0 g/L (100 mg/dL).2-4 Since the disease is infrequent, its natural history is uncertain, although it is widely accepted that sudden death and cardiac block are significant determinants of survival in these patients. For this reason, several groups propose prophylactic implantation of a definitive pacemaker without performing an electrophysiological study in patients with KSS and bifascicular blocks.5 In this article, we report on cardiac involvement and clinical follow-up of a group of patients with KSS.
PATIENTS AND METHODS
All patients in whom KSS was suspected were assessed by a multidisciplinary team to establish a clinical diagnosis of KSS. Once the diagnosis was confirmed, the patients underwent 12-lead surface electrocardiography (ECG), transthoracic echocardiography, 24-hour Holter monitoring and, in patients at high risk for the development of complete atrioventricular block, i.e., presence of bifascicular block, electrophysiological study. All patients underwent striated muscle biopsy for ragged red fibers4-6 by modified trichrome (Engel) stain,6 in addition to ultrastructural analysis with electronic microscopy.
RESULTS
We studied five unrelated patients (3 women, 2 men) with a mean age of 31.6±5.6 years at the time of diagnosis and a mean time of evolution of 21.8±6.8 years. The clinical characteristics are shown in Table. The main symptoms leading to the initial consultation were: asthenia, weight loss, palpitations, and diplopia.
Electrocardiography
In all patients, we found conduction disturbances consisting of bifascicular block (right bundle branch block and left anterior hemiblock) in 2 patients, intermittent periods of 2:1 atrioventricular block (AVB) in 1 patient, left anterior hemiblock in 1 patient, and right bundle branch block in 1 patient.
Electrophysiological Study
Electrophysiological study was indicated in 3 patients (Cases 1-3). The results were normal except for the patient with intermittent periods of 2:1 AVB (Case 3), in whom a definitive pacemaker was implanted. The measurements obtained for the three patients were: baseline cycle length 962, 979, and 1761 ms (mean, 1234±456 ms), atrio-His (A-H) interval of 79, 116, and 80 ms (mean, 91.6±21 ms), and His-ventricular (H-V) interval of 49, 51, and 90 ms (mean, 63.33±23 ms; normal <55 ms). Sinus node recovery time was 395, 225, and 1500 ms, respectively, with a mean of 706.6±692.3 ms (Table 1).
Holter Monitoring
In 1 patient we detected asymptomatic nonsustained monomorphic ventricular tachycardia, and in another patient, episodes of inappropriate sinus tachycardia that produced few symptoms and did not limit functional class.
Echocardiogram
We defined diastolic dysfunction as the delay in isovolumetric relaxation time of the left ventricle (≥100 ms)7 and inversion of the E/A ratio on pulsed Doppler,7 with only one patient (Case 4) found to have this alteration.
We also observed mitral valve prolapse (MVP) with mild mitral regurgitation in two patients, and mitral valve thickening in 2 patients. All other parameters assessed by echocardiography in the series were normal (ejection fraction 54±6%; shortening fraction 29±4%; left ventricular diastolic diameter 35.5±6.8 mm; and left ventricular systolic diameter 23.5±5.3 mm).
Histopathology
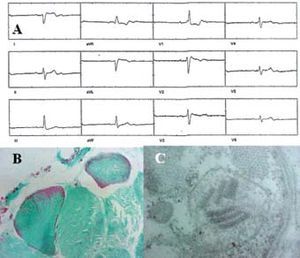
In all patients we found ragged red fibers, considered to be a marker of biochemical damage in oxidative phosphorylation (Figure, B). All patients also had ultrastructural mitochondrial abnormalities, with the most relevant being giant mitochondria and fewer numbers of mitochondria, subsarcolemmal mitochondrial clusters, altered morphology with no cristae and a rounded appearance, and presence of Type I and II intramitochondrial inclusions (Figure, C).
Figure. A. Surface electrocardiogram (Case 3) which reveals 2:1 atrioventricular block. B. Light-field photomicrography of striated muscle (Case 1) with modified trichrome (Engel) stain showing the presence of ragged red fibers; normal muscle is shown in green. C. Transmission electron micrography of muscle biopsy (Case 5) showing megamitochondria in the subsarcolemma; intramitochondrial cristae loss and the presence of "parking lot" paracrystalline inclusions are evident.
Follow-up
At the time of writing, the duration of follow-up was 5.3±1.2 years after the diagnosis. Follow-up consisted of frequent medical examination, including serial ECG and echocardiography, and cardiac rehabilitation in the case of 3 patients. No new blocks or manifestations of cardiac involvement were observed in our series during follow-up.
DISCUSSION
The development of serious cardiac complications is frequent in KSS. According to Berenberg et al,8 clinical manifestations of heart disease occur in 57% of KSS patients and include syncope in 45% of the cases,8 sudden death in 23%,8,9 and cardiomyopathy in 20%.6 Unlike other series, sudden death or clinical or subclinical evidence of cardiomyopathy have not been observed in any of our patients.
An association between KSS and tricuspid valve prolapse and MVP10-13 as well as mitral valve thickening has been established, in which the latter may or may not be related to the MVP.11,12 The mitral thickening is similar to that observed in lupus and phospholipid syndrome, although there are no vegetations, commissure fusion or nodularities in KSS.5,12 However, the pathophysiology of valve involvement in KSS has not been established to date. In our series, 2 patients had MVP, 1 of them with mitral valve thickening, and 1 patient had thickening without MVP but with mild mitral regurgitation.
In comparison with the general population, patients with KSS and bifascicular block are at greater risk of developing complete AV block.5,7 Because KSS patients die suddenly in 23% of cases, a fact attributed to complete cardiac block, several groups have suggested that a prophylactic pacemaker should be implanted whenever a bifascicular block develops, without performing an electrophysiological study.
The clinical progress of patients with KSS varies considerably. The time until patients develop a bifascicular block is uncertain and the interval between this occurrence and the development of complete atrioventricular block or sudden death also unpredictable. There are no clinical data that allow this complication to be anticipated, and although prophylactic pacemaker implantation was previously recommended in patients with KSS and bifascicular block, we believe that electrophysiological study should be compulsory among these patients.
LIMITATIONS
Because this series is small, the stable evolution observed in cardiac involvement among our patients cannot be extrapolated to other groups. Our series includes fewer patients with cardiomyopathy than has been reported in the literature, although factors such as the idiosyncrasy and differences in the degree of segregation, heteroplasmy and genetics could be related to this observation.
CONCLUSIONS
Cardiac involvement in KSS is frequent, although the clinical presentation and the severity may vary. Moreover, cardiac conduction defects play an important role in the clinical condition of these patients. A better understanding of KSS will help in the design of improved therapeutic strategies, such as gene therapy or mitochondrial gene replacement in human cells.14 According to Luft, "Mitochondrial medicine is an expanding discipline, and a greater understanding of this interesting family of diseases should increase the possibility that these patients are appropriately diagnosed and treated."11
Correspondence: Dr. C.F. Barrera-Ramírez.
Departamento de Cardiología Intervencionista. Centro Hospitalario La Concepción.
Blvd. V. Carranza 4036; Col Villa Olímpica. 25230. Saltillo, Coah. México.
E-mail: carlosfbarrera@yahoo.com
Received May 27, 2004.
Accepted for publication September 30, 2004.