To assess the predictive value of atrial natriuretic peptide, β-type natriuretic peptide, copeptin, mid-regional pro-adrenomedullin (MR-proADM) and cardiac troponin I (cTn-I) as indicators of low cardiac output syndrome in children with congenital heart disease undergoing cardiopulmonary bypass (CPB).
MethodsAfter corrective surgery for congenital heart disease under CPB, 117 children (aged 10 days to 180 months) were enrolled in a prospective observational pilot study during a 2-year period. The patients were classified according to whether they developed low cardiac output syndrome. Biomarker levels were measured at 2, 12, 24, and 48 hours post-CPB. The clinical data and outcome variables were analyzed by a multiple logistic regression model.
ResultsThirty-three (29%) patients developed low cardiac output syndrome (group 1) and the remaining 84 (71%) patients were included in group 2. cTn-I levels >14 ng/mL at 2hours after CPB (OR, 4.05; 95%CI, 1.29-12.64; P=.016) and MR-proADM levels>1.5 nmol/L at 24hours following CPB (OR, 15.54; 95%CI, 4.41-54.71; P<.001) were independent predictors of low cardiac output syndrome.
ConclusionsOur results suggest that cTn-I at 2hours post-CPB is, by itself, an evident independent early predictor of low cardiac output syndrome. This predictive capacity is, moreover, reinforced when cTn-I is combined with MR-proADM levels at 24hours following CPB. These 2 cardiac biomarkers would aid in therapeutic decision-making in clinical practice and would also enable clinicians to modify the type of support to be used in the pediatric intensive care unit.
Keywords
Low cardiac output syndrome (LCOS) is a well-recognized entity occurring in 25% to 60% of pediatric patients undergoing open heart surgery with an accepted collection of hemodynamic and physiologic disturbances.1 The onset of LCOS follows a predictable course in the hours following cardiopulmonary bypass (CPB), as myocardial performance declines in the face of an elevated demand for cardiac output. When demand outstrips supply, shock ensues and early recognition and intervention can decrease morbidity and mortality.2
A wide variety of cardiac biomarkers have been reported in adults with heart failure. However, the behavior of cardiac biomarkers—mid-regional-regional pro-atrial natriuretic peptide, β-type natriuretic peptide (BNP), copeptin, mid-regional-regional pro-adrenomedullin (MR-proADM) and cardiac troponin I (cTn-I)—in the postoperative period following cardiac surgery under CPB in children with congenital heart disease has seldom been studied. There have been studies involving children with acute heart failure,3 although most have focused on children with chronic heart failure.4 These reports have noted positive correlations between plasma ANP and BNP levels and the degree of heart failure.5 Currently, BNP and N-terminal-pro-brain natriuretic peptide appear to be prognostic markers of heart failure.
Copeptin, which is more stable than arginine vasopressin peptide, is a prognostic marker of acute myocardial infarction and of acute and chronic cardiac failure in adult patients.6,7 cTn-I and troponin T are highly sensitive and specific biochemical markers of myocardial injury and their levels are associated with hemodynamic status after cardiac surgery in neonates and children.8 Some authors have reported correlations between cTn-I levels and LCOS. They may also predict early in-hospital outcomes and can be useful for identifying young children at increased risk for LCOS and death after heart surgery under CPB.9
Adrenomedullin (ADM) is reportedly associated with the pathophysiology of LCOS. ADM is a prognostic indicator of chronic heart failure in adults, as well as of acute destabilized and ischaemic left ventricular dysfunction.10,11 In addition, high levels of ADM predict long-term adverse clinical events after myocardial injury. However, ADM has barely been studied in the pediatric population.
The objective of this study was to determine whether any of the above-mentioned cardiac biomarkers correlate with the hemodynamic status of children after corrective surgery for congenital heart disease under CPB. If such a relationship were established, the cardiac biomarkers in question could be used as a prognostic maker of LCOS. These cardiac biomarkers would assist in therapeutic decision-making in clinical practice in the pediatric intensive care unit immediately after cardiac surgery under CPB.
METHODSStudy Design: PopulationThis prospective observational pilot study was conducted at a single referral hospital during a 2-year period. The study enrolled 117 consecutive patients (aged 10 days to 180 months) admitted to the pediatric intensive care unit after corrective surgery for congenital heart disease under CPB. The exclusion criteria included: a) infection, b) renal failure, c) polymalformative syndromes, d) congenital metabolic defects, and e) chronic diseases. Patients who died during surgery or within the first 6hours after surgery were also excluded from the study.
Following pediatric intensive care unit admission, the management and monitoring of the patients were performed in accordance with a specific protocol for each type of congenital heart disease. Postoperative hemodynamic monitoring included: a) insertion of an arterial line: a PiCCO catheter was placed into 1 femoral artery. For patients weighing 5 to 15kg, a 3-F catheter (PV 2013 L07 Pulsiocath) was used and a 4-F catheter (PV 2014 L08 Pulsiocath, Pulsion Medical Systems AG, Munich, Germany) was inserted in patients who weighed more than this body-weight threshold. Because of the problems that catheterization may cause, we dispensed with the placement of a PiCCO catheter in the patients weighing<5kg; b) insertion of a central venous catheter (tip either in or close to the right atrium), which was checked by a chest radiograph, and c) occasionally, another catheter was placed into the left atrium of the patients at risk of postoperative left ventricular dysfunction. All patients received mechanical ventilation and were sedated with midazolam and fentanyl.
After corrective surgery, the patients were divided into 2 groups according to the presence (group 1) or absence (group 2) of LCOS. LCOS was diagnosed when the following 2 major criteria were met: a) left ventricular ejection fraction<40% (Teicholz) via echocardiography, and b) cardiac index<2.5 L/min/m2 via an arterial thermodilution catheter (PiCCO) in all the children weighing ≥ 5kg. LCOS was considered in children weighing<5kg when they exhibited ventricular ejection fraction<40% (left ventricle, with the exception of hypoplastic left heart syndrome, in which the right ventricle functions as the systemic ventricle), and they met at least 3 of the following minor criteria: a) systolic blood pressure<p5 for age and sex, b) urine output<1mL/kg/h without diuretics, c) lactate ≥ 3.5 mmol/L or HCO3<18 mEq/L, d) inotropic score ≥ 20,12 and e) oxygen extraction ratio>35%.
Blood SamplingBaseline blood samples were collected from a central venous catheter prior to corrective surgery and at 2, 12, 24, and 48hours post-CPB. Assessment of the patient's general biochemical parameters and quantification of BNP and cTn-I levels were carried out in our department of clinical analysis. Blood samples were collected and centrifuged at 3500g for 10minutes. Serum was separated into aliquots and frozen at –82°C until the day of analysis.
BNP and cTn-I were measured using an automated electrochemiluminescence immunoassay (Architect system, Abbott Diagnostics Division, Illinois, United States). The Architect BNP assay was designed with a ≤ 10 pg/mL detection limit. The detection limit of the ARCHITECT STAT troponin I assay was ≤ 0.01 ng/mL. N-terminal pro-atrial natriuretic peptide, MR-proADM, and copeptin were measured using a simultaneous multianalyte detection immunoassay BRAHMS KRYPTOR compact (Hennigsdorf, Germany), as well as assay kits and a Multiplex system using Luminex xMAP technology. The analytical detection limits were 0.05 nmol/L for MR-proADM, 4.8pmol/L for copeptin, and 2.1pmol/L for N-terminal pro-atrial natriuretic peptide.
Statistical AnalysisData are expressed either as mean±standard deviation or as median (interquartile ranges). Comparisons between group 1 and group 2 were undertaken using the Student t test for continuous variables when the distribution was normal (Kolmogorov-Smirnov test) and the Mann-Whitney U test when the distribution was not normal. The relationships between the categorical variables were assessed using either the chi-square test or the Fisher exact test. Comparisons among the monitored time points were tested via a Mixed-designs ANOVA test and a Sidak correction as a post-hoc test. The data were adjusted for the age and body weight of the patients, using age and body weight as covariates. The cTn-I analysis was adjusted for aortic cross clamping and CPB time.
The correlations between the peak postoperative values of each of the biomarkers listed above were assessed via the Spearman correlation test. Univariate logistic regression was undertaken at 48hours following surgery to identify the independent predictors of LCOS. The relationships between the variables were assessed via an odds ratio with 95% confidence interval. In the multivariate analysis, the independent predictors of LCOS were considered risk factors (P<.05), based on the results of the univariate analysis. The Hosmer-Lemeshow test and the receiver operating characteristic curves were applied to assess the goodness-of-fit of the model. The analysis of the receiver operating characteristic curves and the area under the curve was generated with 95% confidence intervals to determine the cutoff points for cTn-I and MR-proADM, which might have provided a more accurate identification of the LCOS patients. Sensitivity, specificity, positive and negative predictive values for MR-proADM, cTn-I, and the combination of both biomarkers were also calculated using the marker-specific cutoffs. All the tests were 2-tailed and the results were considered statistically significant when P<.05. The data were analyzed using SPSS 18.0.0 2010 (SPSS Inc., Chicago, Illinois, United States).
Patient Medical ReportsData were obtained from the case history of each child. Informed written consent was obtained from the patients’ parents or legal guardians. Our study was approved by the Hospital Biomedical Ethics Committee and conformed to the ethical standards laid down in the 1964 Declaration of Helsinki.
RESULTSOne hundred seventeen congenital heart disease patients aged 10 days to 180 months (mean 39.3±52.4 months) were enrolled in the present study: 19 infants (16%) were younger than 30 days.
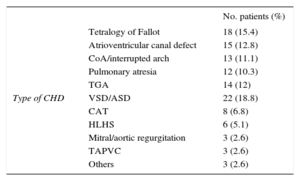
The patient's type of congenital heart disease is described in Table 1. Thirty-three (29%) patients developed LCOS (group 1) after corrective surgery and the remaining 84 (71%) patients were included in Group 2. Six patients (2 neonates) developed cardiogenic shock and required extracorporeal membrane oxygenation within the first 48hours following CPB. One patient received extracorporeal membrane oxygenation in the operating theatre, 2 within the first 12hours and 3 patients 24 to 48hours postoperatively.
Patient Description by Type of Congenital Heart Disease
| No. patients (%) | ||
|---|---|---|
| Type of CHD | Tetralogy of Fallot | 18 (15.4) |
| Atrioventricular canal defect | 15 (12.8) | |
| CoA/interrupted arch | 13 (11.1) | |
| Pulmonary atresia | 12 (10.3) | |
| TGA | 14 (12) | |
| VSD/ASD | 22 (18.8) | |
| CAT | 8 (6.8) | |
| HLHS | 6 (5.1) | |
| Mitral/aortic regurgitation | 3 (2.6) | |
| TAPVC | 3 (2.6) | |
| Others | 3 (2.6) |
ASD, atrial septal defect; CAT, common arterial trunk; CHD, congenital heart disease; CoA, coarctation of the aorta; HLHS, hypoplastic left heart syndrome; TAPVC, total anomalous pulmonary venous connection; TGA, transposition of the great arteries; VSD, ventricular septal defect.
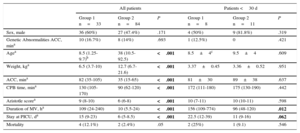
The characteristics of all the patients and the neonates<30 days, as well as the surgery data and the classification of the patients into groups according to the presence or absence of LCOS after CPB are shown in Table 2. The children in group 1 were younger and weighed less than the children in group 2. The duration of aortic cross-clamping and CPB was prolonged in group 1 (P<.001). The sex distribution of the 2 groups was homogeneous.
Patient Characteristics in the Study Groups
| All patients | Patients <30 d | |||||
|---|---|---|---|---|---|---|
| Group 1 n=33 | Group 2 n=84 | P | Group 1 n=8 | Group 2 n=11 | P | |
| Sex, male | 36 (60%) | 27 (47.4%) | .171 | 4 (50%) | 9 (81.8%) | .319 |
| Genetic Abnormalities ACC, mina | 10 (16.7%) | 8 (14%) | .693 | 1 (12.5%) | 0 | .421 |
| Agea | 8.5 (1.25-9.7)b | 38 (10.5-92.5) | <.001 | 8.5±4c | 9.5±4 | .609 |
| Weight, kga | 6.5 (3.7-10) | 12.7 (6.7-21.6) | <.001 | 3.37±0.45 | 3.36±0.52 | .951 |
| ACC, mina | 82 (35-105) | 35 (15-65) | <.001 | 81±30 | 89±38 | .637 |
| CPB time, mina | 130 (105-170) | 90 (62-120) | <.001 | 172 (111-180) | 175 (130-190) | .442 |
| Aristotle scorea | 9 (8-10) | 6 (6-8) | <.001 | 10 (7-11) | 10 (10-11) | .598 |
| Duration of MV, ha | 109 (24-240) | 10 (5.5-24) | <.001 | 156 (109-774) | 96 (48-120) | .012 |
| Stay at PICU, da | 15 (9-23) | 6 (5-8.5) | <.001 | 22.5 (12-39) | 11 (9-16) | .062 |
| Mortality | 4 (12.1%) | 2 (2.4%) | .05 | 2 (25%) | 1 (9.1) | .546 |
ACC, aortic cross-clamping; CPB, cardiopulmonary bypass; MV, mechanical ventilation; PICU, pediatric intensive care unit. Group 1: Patients with low cardiac output syndrome; group 2: Patients without low cardiac output syndrome.
P values in bold are statistically significant.
In all patients, comparisons of baseline levels of the cardiac biomarkers (median and interquartile range) with their levels at 2hours post-CPB showed statistically significant increases in cTn-I levels: 12.3 (4.6-36.1) vs 0.02 (0-0.04) ng/mL; P<.001; MR-proADM, 2.24 (1.34-3.26) vs 0.51 (0.35-0.99) nmol/L; P<.001; and copeptin levels, 132.8 (46.64-309.5) vs 6.9 (4.7-28.5) pmol/L; P<.001. BNP peaked at 12hours of CPB (441.4 [241.9-1023.5] pg/mL vs 50.5 [25.7-186.15] pg/mL, P=.020). N-terminal pro-atrial natriuretic peptide concentrations remained unchanged compared with their baseline values.
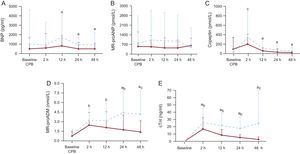
cTn-I was the earliest biomarker of LCOS that showed significant differences between group 1 and group 2 at 2hours after heart corrective surgery. Copeptin and BNP values had a similar course in the 2 groups. However, after attaining a peak value at 2hours postoperatively, cTn-I and MR-proADM levels decreased in group 2 but increased in group 1 (Figure 1). Postoperative biomarkers in neonates showed a similar profile to those in the rest of the patients (Figure 2)
Changes in biomarker levels over time for all the study patients stratified according to the presence or absence of postoperative heart failure. A: BNP. B: MR-proANP. C: copeptin. D: MR-proADM. E: cTn-I levels. BNP, β-type natriuretic peptide; CPB, cardiopulmonary bypass; cTn-I, cardiac troponin-I; MR-proADM, mid-regional pro-adrenomedullin; MR-proANP, mid-regional pro-atrial natriuretic peptide.
----- Group 1
— Group 2
•Represents the mean, and error bars indicate the standard deviation.
aSignificant differences between group 1 and group 2.
bSignificant differences vs baseline in both groups (P <.05).
cSignificant differences vs baseline in group 1 (P <.05).
Changes in biomarker levels over time for patients younger than 30 days stratified according to the presence or absence of postoperative heart failure. A: BNP. B: MR-proANP. C: copeptin. D: MR-proADM. E: cTn-I levels. BNP, β-type natriuretic peptide; CPB, cardiopulmonary bypass; cTn-I, cardiac troponin-I; MR-proADM, mid-regional pro-adrenomedullin; MR-proANP, mid-regional pro-atrial natriuretic peptide.
----- Group 1
— Group 2
•Represents the mean, and error bars indicate the standard deviation.
aSignificant differences between group 1 and group 2.
bSignificant differences vs baseline in both groups (P <.05).
cSignificant differences vs baseline in group 1 (P <.05).
BNP, N-terminal pro-atrial natriuretic peptide, MR-proADM, and copeptin remained unchanged at the different monitoring time points compared with their baseline levels in the 6 patients requiring extracorporeal membrane oxygenation. However, cTn-I levels peaked at 2hours after surgery: 19.33 (3.7-32.5) vs 0.02 (0.01-0.03 ng/mL; P<.001), but remained without significant changes in the next postoperative time points.
CorrelationsThe peak cTn-I values at 2hours correlated with CPB time (r=0.408; P<.001). However, the peak MR-proADM values inversely correlated with patient age (r=-0.560; P<.001) and directly correlated with CPB time (r=0.408; P<.001) and the pediatric intensive care unit discharge (r=0.490; P<.001).
There was a positive association between MR-proADM plasma levels (48hours after surgery) with lactate levels in group 1 (r=0.572, P<.001) but not in group 2 (r=0.247, P<.12). We found no correlation between the oxygen extraction ratio and MR-proADM levels in the 2 groups. Likewise, no correlation was found between cTn-I and lactate levels or the oxygen extraction ratio.
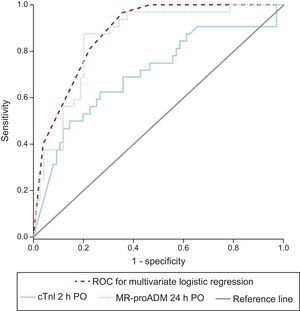
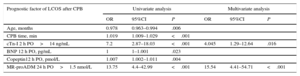
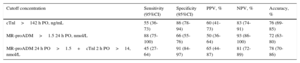
Logistic Regression AnalysisTable 3 describes the most significant parameters obtained via the univariate and multivariate logistic regression analyses in relation to the prediction of LCOS after CPB. cTn-I levels (> 14 ng/mL) at 2hours following heart corrective surgery under CPB and MR-proADM levels (> 1.5 nmol/L) at 24hours postoperatively were independent predictors of LCOS. The logistic regression results and the areas under the curve of cTn-I and MR-proADM are depicted in Figure 3. Comparisons among the areas under the curve of these biomarkers showed no statistically significant differences. Sensitivity, specificity, and positive and negative predictive values were calculated at cutoff values of 14 ng/mL for cTn-I and 1.5 nmol/L for MR-proADM (Table 4). The combination of MR-proADM and cTn-I had high specificity and provided more accurate detection of LCOS than the use of MR-proADM alone.
Univariate and Multivariate Logistic Regression for the Prediction of Low Cardiac Output Syndrome After Cardiac Surgery Under Cardiopulmonary Bypass
| Prognostic factor of LCOS after CPB | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |
| Age, months | 0.978 | 0.963–0.994 | .006 | |||
| CPB time, min | 1.019 | 1.009–1.029 | <.001 | |||
| cTn-I 2 h PO>14 ng/mL | 7.2 | 2.87–18.03 | <.001 | 4.045 | 1.29–12.64 | .016 |
| BNP 12 h PO, pg/mL | 1 | 1–1.001 | .023 | |||
| Copeptin12 h PO, pmol/L | 1.007 | 1.002–1.011 | .004 | |||
| MR-proADM 24 h PO>1.5 nmol/L | 13.75 | 4.4–42.99 | <.001 | 15.54 | 4.41–54.71 | <.001 |
95%CI, 95% confidence interval; BNP, β-type brain natriuretic peptide; CPB, cardiopulmonary bypass; cTn-I, cardiac troponin I; LCOS, low cardiac output syndrome; MR-proADM, mid-regional pro-adrenomedullin; OR, odds ratio; PO, postoperative.
Hosmer-Lemeshow test=2.93; P=.711.
Receiver operating characteristic curves for the diagnosis of low cardiac output syndrome at 48hours PO. The area under the curve of MR-proADM at 24hours PO was 0.848 (95%CI, 0.771-0.924); area under the curve of cTn-I at 2hours PO was 0.702 (95%CI, 0.581-0.812); area under the curve of cTn-I>14 ng/mL at 2hours PO+MR-proADM>1.5 mmol/L at 24hours PO was 0.885 (95%CI, 0.823-0.947). cTn-I, cardiac troponin I; MR-proADM, mid-regional pro-adrenomedullin; PO, postoperative; ROC, receiver operating characteristic.
Diagnostic Information for the Prediction of Low Cardiac Output Syndrome Via Cardiac Troponin I and Mid-regional Pro-adrenomedullin Levels
| Cutoff concentration | Sensitivity (95%CI) | Specificity (95%CI) | PPV, % | NPV, % | Accuracy, % |
|---|---|---|---|---|---|
| cTnl>142 h PO, ng/mL | 55 (36-73) | 86 (78-94) | 60 (41-73) | 83 (74-91) | 76 (69-85) |
| MR-proADM>1.5 24 h PO, nmol/L | 88 (75-100) | 66 (55-76) | 50 (36-64) | 93 (86-100) | 72 (63-80) |
| MR-proADM 24 h PO>1.5+cTnl 2 h PO>14, nmol/L | 45 (27-64) | 91 (84-97) | 65 (44-87) | 81 (72-89) | 78 (70-86) |
95%CI, 95% confidence interval; cTn-I, cardiac troponin I; MR-proADM, mid-regional pro-adrenomedullin; NPV, negative predictive value; PO, postoperative; PPV, positive predictive value.
Monitoring cardiac biochemical markers after corrective surgery is highly effective in adults with heart failure.4 However, there are few studies involving children after corrective surgery under CPB to establish their effectiveness.1,3 The present prospective observational pilot study was conducted with a large sample size and with minimal variability for patient management. We observed that the prediction of LCOS was strengthened by a cutoff point of>14 ng/mL for cTn-I at 2hours post-CPB, regardless of the age of the patients, the physiological heart defect, and the duration of CPB. cTn-I is a specific and sensitive marker of myocardial injury after cardiac surgery and it may predict early in-hospital outcomes. The prediction of LCOS is, by itself, evident but it was reinforced when cTn-I values at 2hours following CPB were combined with a cutoff point of>1.5 nmol/L for MR-proADM at 24hours after CPB. Levels of BNP, N-terminal pro-atrial natriuretic peptide and copeptin were not found to be early predictors of LCOS in the early hemodynamic monitoring of the patients.
LCOS is defined as the inability of the heart to maintain adequate minute volume to provide oxygen and nutrients to the tissue. This concept involves the influence of cardiac output and peripheral vessels on resistance to blood flow and tissue perfusion. Usually, decreased ventricular function reaches a peak between 8 and 12hours and recovers gradually at 24 to 48hours following CPB.1 In addition to routine monitoring methods, ventricular function may also be assessed by analyzing cardiac biomarkers.
There are few studies of the reference intervals for biomarker assays in the pediatric population. Moreover, there are no data on the reference levels of copeptin and MR-proADM in children. In our study, all the data were adjusted for the patients’ age and bodyweight to prevent these variables from influencing our results. Although no studies have reported sex-related alterations in cardiac biomarkers, the sex distribution in our study was homogenous.
Elevation of plasma levels of the studied biomarkers, regardless of whether the patients had LCOS, suggested that important alterations occur in children exposed to CPB during surgical repair. Some studies have observed an increase in N-terminal pro-BNP levels and few changes in N-terminal pro-atrial natriuretic peptide.9,13 The plasma levels of these 2 cardiac biomarkers appear to vary depending on the patient's hemodynamic status and on the characteristics of congenital heart disease.14
BNP is eliminated through the hemofiltration process, akin to ultrafiltration mechanisms, in the last stage of CPB.15 Consequently, the biological activity of the natriuretic hormone system may decrease and the infusion of natriuretic hormones might be clinically beneficial.16,17 Several studies have noted an association between an increase in N-terminal pro-BNP and a higher incidence of complications in the postoperative period.16 Carmona et al.9 have reported a correlation between LCOS and N-terminal pro-BNP levels preoperatively. In contrast, in our study, no useful tool was provided for identifying children at increased risk of LCOS following corrective surgery. There are no studies on postoperative levels of copeptin in children undergoing cardiac surgery. In the present study, serial measurement of copeptin proved to be ineffective.
cTn-I and troponin T indicate the presence of injury-associated necrosis of myocardial cells. The levels of cTnl increase rapidly after cardiac injury and could serve as a predictive cardiac biomarker during the postoperative course of patients with congenital heart disease undergoing cardiac surgery.9,18 Previous studies have indicated that cTn-I levels at 4hours post-CPB enable clinicians to predict the postoperative course of pediatric patients after cardiac surgery.19 Bojan et al.20 have determined that an early increase in cTn-I levels can be useful in predicting the postoperative course of neonates and infants undergoing cardiac surgery. Moreover, the duration of aortic clamping may stimulate cTn-I levels to increase.21 Using a cutoff point of 13 ng/mL at 4hours post-CPB, Froese et al.22 have demonstrated that the early measurement of cTn-I can enable clinicians to recognise the development of LCOS after cardiac surgery in children. In contrast, a cutoff point of>14 ng/mL for cTn-I at 2hours post-CPB in the present study showed high sensitivity and specificity. cTn-I is a specific and sensitive marker of myocardial injury after cardiac surgery and is, by itself, an evident independent early predictor of LCOS, regardless of patient age, the physiological heart defect, and the duration of CPB.
Little research has been conducted on the effects of CPB on plasma MR-proADM levels. ADM is difficult to measure in plasma; however, MR-proADM is more stable than the active molecule, being secreted in equimolar amounts to ADM. Circulating ADM levels are increased in heart failure and are related to low left ventricular ejection fraction. Hence, ADM secretion appears to be a compensatory mechanism in LCOS, which also plays an important role in fluid homeostasis.
Our findings differ from those reported by Abella et al.,23 who have described an initial decrease in ADM levels during and after CPB in LCOS children. Takeuchi et al.24 have also noted a decrease in ADM plasma levels at 24hours following CPB. Both studies were conducted with a limited number of patients; therefore, these results should be interpreted with caution. Accordingly, it seems plausible that in most critical patients ADM levels could be increased. The increase in ADM in LCOS could be a compensatory mechanism which increases cyclic adenosine monophosphate levels, activates protein kinase A, and stimulates myocardial contractility, coronary flow, and cardiac output.23 Given the small number of patients requiring extracorporeal membrane oxygenation in our study, we deemed it inappropriate to conduct a statistical analysis of the studied cardiac biomarkers. The transit of blood through the extracorporeal circuit may result in the degradation and/or sequestration of these biomarkers. As reported by Luyt et al.,25 monitoring these cardiac biomarkers in extracorporeal membrane oxygenation patients would not aid therapeutic decision-making.
Postoperative measurement of cTn-I and MR-proADM, besides the use of hemodynamic monitoring and markers of tissue perfusion, could be used in congenital heart disease patients to determine the risk of LCOS after corrective surgery under CPB. Aside from the monitoring of hemodynamic and respiratory parameters, serum lactate monitoring and continuous near-infrared spectroscopy measurement of tissue oxygen saturation are essential to detect LCOS. Research into the levels of these biomarkers may also help in the evaluation of cardiac output and tissue oxygenation. This would facilitate therapeutic decision-making in clinical practice and would enable clinicians to modify the type of support. The analysis of the studied cardiac biomarkers at 2 and 24hours after surgery cannot be considered separately but should be complemented with data further monitoring, which is usually obtained postoperatively.
LimitationsThe limitations of the current study mainly involve the genomic aspects of the cardiac biomarkers, the interactions among these biomarkers, which can be complex, and the fact that individual variations in these biomarkers can be large. However, these aspects have not yet been sufficiently studied. In the multivariate analysis, there was a wide range of confidence intervals for odds ratios. This factor is outside our influence and it mainly depends on variability in the measurements. Some relevant variables that may influence LCOS were not included in the multivariate analysis. The variability in the clinical postoperative complexity of patients undergoing CPB, as well as the surgical procedures, make it difficult to perform an exhaustive monitoring of all the possible variables predictive of LCOS. However, we previously conducted a descriptive and a univariate analysis to prevent such variability. The validity of our results is also supported by the high values obtained for odds ratios.
The major strengths of this study are its large sample size and the minimal variability in patient management. LCOS was considered based upon restrictive and objective inclusion criteria.
CONCLUSIONSOur study demonstrated that LCOS prediction at 48hours was strengthened by a cutoff point of>14 ng/mL for cTn-I at 2hours post-CPB. cTn-I is a specific and sensitive marker of myocardial injury after cardiac surgery. The early prediction of LCOS is, by itself, evident, regardless of the physiological heart defect and the duration of CPB. This result was reinforced when cTn-I values at 2hours following CPB were combined with a cutoff point of>1.5 nmol/L for MR-proADM at 24hours after CPB. Levels of these cardiac biomarkers, combined with monitoring of hemodynamic and respiratory parameters, could provide complementary information to aid in therapeutic decision-making and prevent hemodynamic deterioration in the immediate postoperative period in congenital heart disease.
- -
A wide variety of cardiac biomarkers has been reported in adults with heart failure. The behavior of cardiac biomarkers in the postoperative period following cardiac surgery under CPB in children with congenital heart disease has seldom been studied.
- -
If any of the studied cardiac biomarkers correlated with the hemodynamic status of the children after corrective surgery for congenital heart disease, the cardiac biomarker in question could be used in therapeutic decision-making in clinical practice after surgery.
- -
The prediction of LCOS was strengthened by a cutoff point of>14 ng/mL for cTn-I at 2hours post-CPB. The early prediction of LCOS is, by itself, evident, regardless of the physiological heart defect and the duration of CPB. This result was reinforced when cTn-I values were combined with a cutoff point of>1.5 nmol/L for MR-proADM at 24hours post-CPB.
- -
Levels of these cardiac biomarkers could provide complementary information to aid in therapeutic decision-making and to prevent hemodynamic deterioration in the immediate period post-CPB.
This study was supported by BRAHMS GmbH Biotechnology Centre, Hennigsdorf, Berlin, Germany. The sponsor of the study had no role in the study design or in data collection, analysis, and interpretation and did not participate in writing the report.
CONFLICTS OF INTERESTNone declared.