High-sensitivity troponin T assays (Hs-TnT) have been carried out in selected populations in clinical trials and in registries of the general population with low cardiovascular risk (CVR). The aim of this study was to determine the proportion of individuals with detectable Hs-TnT and the proportion of individuals with elevated Hs-TnT in a Spanish population of asymptomatic individuals with very high CVR, as well as the parameters associated with Hs-TnT elevation.
MethodsThe study included 690 patients. Hs-TnT detection and Hs-TnT elevation (≥99th percentile value), as well the association of elevated Hs-TnT and clinical, analytical, and treatment data were analyzed.
ResultsHs-TnT was analyzed in 646 patients and was detected in 645. Elevated TnT was detected in 212 patients (32.9%). On multivariate analysis, elevated TnT was independently associated with male sex (OR, 2.81; 95%CI, 1.67-4.73; P < .001), older age (OR, 1.06; 95%CI, 1.04-1.09; P < .001), a higher body mass index (OR, 1.07; 95%CI, 1.02-1.12; P < .002), insulin therapy (OR, 1.99; 95%CI, 1.15-3.46; P = .01), history of heart failure (OR, 3.92; 95%CI, 1.24-12.39; P = .02), and estimated glomerular filtration rate calculated by CKD-EPI (OR, 0.96; 95%CI, 0.95-0.97; P < .001).
ConclusionsIn a Spanish population of asymptomatic individuals at very high CVR, Hs-TnT was associated with older age, male sex, higher body mass index, insulin therapy, history of heart failure, and lower glomerular filtration rate.
Keywords
Cardiac troponin is a component of the cardiomyocyte contratile apparatus that is released to the bloodstream after myocardial injury. The high-sensitivity troponin T (Hs-TnT) assay detects smaller amounts of troponin T than conventional assays, permitting earlier and more precise diagnosis and improved treatment.1 However, the use of Hs-TnT assays also increases the proportion of emergency patients and outpatients with detectable levels of cardiac troponin T. High-sensitivity troponin T is detected not only in the acute phase of coronary events, but also in clinical trial patient populations with ischemic heart disease,2 heart failure (HF),3 and kidney disease; in healthy populations with no evident heart disease4–6; and in emergency room patients with no coronary event.7
There is currently no information on the prevalence in Spain of detectable Hs-TnT and elevated Hs-TnT (≥ the 99th percentile value) in asymptomatic individuals at very high cardiovascular risk (CVR) and receiving stable medical treatment. The aim of this study was to determine the proportion of individuals with detectable Hs-TnT and the proportion of individuals with elevated Hs-TnT in a Spanish population of asymptomatic individuals with very high CVR, as well as the parameters associated with Hs-TnT elevation.
METHODSStudy PopulationThe TUSARC registry (Troponina T UltraSensible en pacientes de muy Alto Riesgo Cardiovascular [High-sensitivity troponin T in patients at high cardiovascular risk]) compiles data from a prospective cohort of asymptomatic individuals who meet the criteria for very high CVR according to the previous European Society of Cardiology definition: cardiovascular disease diagnosed by invasive or noninvasive methods; previous myocardial infarction, percutaneous or surgical coronary revascularization, or stroke; peripheral artery disease; type 1 or 2 diabetes mellitus associated with another CVR factor or target organ involvement; SCORE risk ≥ 10%; or glomerular filtration rate (GFR)≤60mL/min/1.73m2.8 The registry was approved by the Burgos University Hospital Ethics Committee (ref. CEIC 1008), and all participants gave written informed consent.
Study participants were recruited during outpatient consultations in the cardiology and internal medicine departments at our hospital. To be included, patients had to have at least 1 of the high CVR factors listed above, have had no treatment modifications in the 3 months preceding inclusion, and be asymptomatic at the time of recruitment. Asymptomatic status was defined as follows: a) absence in the 3 months preceding inclusion of any clinical cardiovascular event (angina, acute myocardial infarction [AMI], cerebrovascular ischemic accident, or clinical HF episode); b) a minimum of 3 months since surgical coronary or peripheral revascularization, and c) a minimum of 6 months since percutaneous coronary revascularization. The 6-month wait period after percutaneous coronary revascularization was chosen because this is the highest risk period for in-stent restenosis. At the time of inclusion, none of the patients was in New York Heart Association functional class III-IV, and none mentioned difficulties with day-to-day activities. A history of clinical HF was identified from a review of clinical event history showing objective evidence of water retention in chest X-ray, a description of edema or symptoms compatible with HF, or clinical improvement after diuretic therapy.
Between March 2013 and March 2015, a total of 690 patients gave informed consent to participate in the study. Of the patients who initally gave consent, 44 did not attend the scheduled visit for blood extraction and were excluded from the analysis. Thus, a total of 646 patients were examined for Hs-TnT and were included in the statistical analysis. For all participants, a specifically designed data sheet was used to record anthropometric data, CVR factors, clinical characteristics, relevant comorbidities, and medical treatment. In addition to Hs-TnT determination, analytical biochemistry parameters were recorded for all patients, including a study of kidney function. Kidney function was assessed from measurements of serum creatine, microalbumin, the albumin-creatine index (determined in first-morning urine samples), and GFR estimated with the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI). Left ventricular hypertrophy (LVH) was diagnosed on electrocardiogram traces according to the Cornell criteria. Peripheral vascular disease and carotid atheromatosis were diagnosed by the presence of ≥ 50% stenosis.
Laboratory ParametersBlood samples were collected in EDTA tubes and centrifuged within 4 hours of collection, and the plasma samples obtained were frozen at –21°C. Batches of frozen plasma samples were sent periodically by specialized messenger service for processing at the central laboratory (Hospital Central de la Defensa Gómez Ulla, Madrid, Spain). In this study, troponin T concentration was measured using the Cobas 6000 platform for high-sensitivity determination, with a minimum detection level of 3 ng/L and a predetermined reference value of 14 ng/L for the 99th percentile in the healthy population. Data quality was ensured by the use of internal and external controls. The internal control was provided by Roche Diagnostics (Preci Control Troponin). The control for low concentrations (lot No. 17568000) had a calculated variation coefficient of 2.4% and the control for high concentrations (lot No. 17405200) had a calculated variation coefficient of 3%. For the external control, we used the Seronorm Immunoassay Liq L-1/Immunoprotein Liq system. Values were assigned according to the stipulations of European directive 98/79/CE on in vitro diagnostic medical devices and international standard ISO 17511. The lot number was 1208448, with a variation coefficient of 5.1%.
Elevated Hs-TnT was defined as a concentration ≥ 14 ng/L.
Statistical AnalysisStatistical analysis was conducted with Stata 13.1 (StataCorp; College Station, Texas, United States). Quantitative variables are presented as mean ± standard deviation and qualitative variables as numbers and frequencies. Quantitative variables were analyzed by the Student t test and qualitative variables by the chi-square test. The adjusted model for the multivariable analysis included all variables found to be significant in the univariable analysis. We calculated the odds ratio (OR), 95% confidence interval (95%CI) and its statistical significance (P value), and the C statistic for the final predictive model. The final predictive model was the equation with largest area under the curve of all possible equations that included the significant variables in the multivariable analysis.
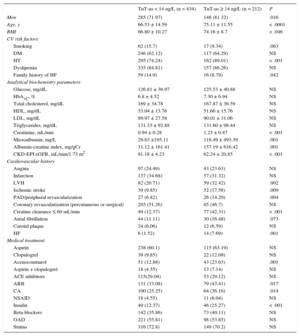
RESULTSUnivariable AnalysisIn total, Hs-TnT was detected in 645 of the 646 patients (99.85%), and was ≥ 14 ng/L (elevated Hs-TnT) in 212 patients (32.9%; 95%CI, 29-36.2). Among patients without elevated Hs-TnT (n = 434), mean Hs-TnT was 7.92 ± 3.11. Among patients with elevated Hs-TnT, mean Hs-TnT was 27.7 ± 38.23. Demographic characteristics, analytical biochemistry variables, and cardiovascular and medical treatment history are listed in Table 1 according to whether patients had elevated or nonelevated Hs-TnT. Patients with elevated Hs-TnT tended to be older and more overweight as assessed by body mass index (BMI), and were predominantly male. In relation to CVR factors, patients with elevated Hs-TnT had higher blood pressure but showed no differences from the other patients in analytical biochemistry parameters except for those related to kidney function: serum creatine, microalbumin, albumin-creatine index, and GFR calculated by CKD-EPI. The elevated Hs-TnT population had more history of clinical HF and higher rates of LVH, ischemic stroke, and peripheral arterial disease/peripheral revascularization. In addition, significantly more patients with elelvated Hs-TnT had a GFR ≤ 60 mL/min/1.73 m2. The 2 patient groups showed no difference in any specifically coronary event (angina AMI, and percutaneous or surgical revascularization). Significant between-group differences were detected in the rates of treatment with acenocoumarol, the use of insulin to control diabetes mellitus, and the use of angiotensin II receptor blockers and calcium antagonists.
Demographic, Clinical, and Treatment Characteristics of Patients Classified According to the Presence or Absence of Elevated Hs-TnT (≥ 99th Percentile Value)
| TnT-us < 14 ng/L (n = 434) | TnT-us ≥ 14 ng/L (n = 212) | P | |
|---|---|---|---|
| Men | 285 (71.97) | 148 (81.32) | .016 |
| Age, y | 66.53 ± 14.59 | 75.11 ± 11.55 | < .0001 |
| BMI | 66.80 ± 10.27 | 74.16 ± 8.7 | < .046 |
| CV risk factors | |||
| Smoking | 62 (15.7) | 17 (9.34) | .063 |
| DM | 246 (62.12) | 117 (64.29) | NS |
| HT | 295 (74.24) | 162 (89.01) | < .001 |
| Dyslipemia | 335 (84.81) | 157 (86.26) | NS |
| Family history of HF | 59 (14.9) | 16 (8.79) | .042 |
| Analytical biochemistry parameters | |||
| Glucose, mg/dL | 126.81 ± 36.97 | 125.53 ± 40.88 | NS |
| HbA1C, % | 6.8 ± 4.52 | 7.30 ± 6.94 | NS |
| Total cholesterol, mg/dL | 169 ± 34.78 | 167.87 ± 36.59 | NS |
| HDL, mg/dL | 53.04 ± 13.76 | 51.66 ± 15.76 | NS |
| LDL, mg/dL | 89.97 ± 27.58 | 90.01 ± 31.06 | NS |
| Triglycerides, mg/dL | 131.33 ± 92.88 | 131.60 ± 98.44 | NS |
| Creatinine, mL/min | 0.94 ± 0.28 | 1.23 ± 0.47 | < .001 |
| Microalbumin, mg/L | 29.83 ±195.11 | 118.49 ± 493.39 | .001 |
| Albumin-creatine index, mg/gCr | 31.12 ± 161.41 | 157.19 ± 616.42 | .001 |
| CKD-EPI eGFR, mL/min/1.73 m2 | 81.18 ± 4.23 | 62.24 ± 20.85 | < .001 |
| Cardiovascular history | |||
| Angina | 97 (24.49) | 43 (23.63) | NS |
| Infarction | 137 (34.68) | 57 (31.32) | NS |
| LVH | 82 (20.71) | 59 (32.42) | .002 |
| Ischemic stroke | 39 (9.85) | 32 (17.58) | .009 |
| PAD/peripheral revascularization | 27 (6.82) | 26 (14.29) | .004 |
| Coronary revascularization (percutaneous or surgical) | 203 (51.26) | 85 (46.7) | NS |
| Creatine clearance ≤ 60 mL/min | 49 (12.37) | 77 (42.31) | < .001 |
| Atrial fibrillation | 44 (11.11) | 30 (16.48) | .073 |
| Carotid plaque | 24 (6.06) | 12 (6.59) | NS |
| HF | 6 (1.52) | 14 (7.69) | .001 |
| Medical treatment | |||
| Aspirin | 238 (60.1) | 115 (63.19) | NS |
| Clopidogrel | 39 (9.85) | 22 (12.09) | NS |
| Acenocoumarol | 51 (12.88) | 43 (23.63) | .001 |
| Aspirin + clopidogrel | 18 (4.55) | 13 (7.14) | NS |
| ACE inhibitors | 115(29.04) | 53 (29.12) | NS |
| ARB | 131 (33.08) | 79 (43.41) | .017 |
| CA | 100 (25.25) | 64 (36.16) | .014 |
| NSAID | 18 (4.55) | 11 (6.04) | NS |
| Insulin | 49 (12.37) | 46 (25.27) | < .001 |
| Beta-blockers | 142 (35.86) | 73 (40.11) | NS |
| OAD | 221 (55.81) | 98 (53.85) | NS |
| Statins | 316 (72.8) | 149 (70.2) | NS |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; BMI, body mass index; CA, calcium antagonist; carotid plaques were defined by the presence of plaques causing > 50% stenosis; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; DM, diabetes mellitus (antidiabetic medication or analytical variables at the time of inclusion); dyslipemia was defined as medication with lipid-lowering agents or LDL > 160 mg/dL; eGFR, estimated glomerular filtration rate; HF, clinical heart failure episode; Hs-TnT, high-sensitivity troponin T; HT, hypertension (medication with antihypertensive drugs or mean blood pressure > 140/90mmHg in 3 consultations); LDL, low-density lipoprotein; LVH, left ventricular hypertrophy detected electrographically according to the Cornell criteria; NSAID, nonsteroidal anti-inflammatory drugs; OAD, oral antidiabetics, PAD, peripheral artery disease (claudication symptoms).
Microalbumin and the albumin-creatine index were determined in first-morning urine samples.
Values are expressed as no. (%) or mean ± standard deviation.
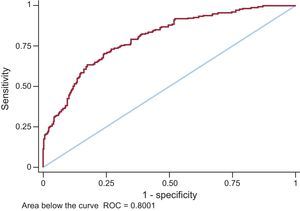
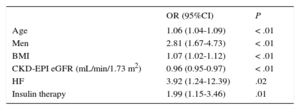
For all variables that were statistically significant in the univariable model (P < .05), a multivariable logistic regression analysis was conducted with elevated Hs-TnT (≥ 14 ng/L) as the dependent variable (Table 2). The only variables showing an independent association with elevated Hs-TnT were age, male sex, BMI, a history of clinical HF, and insulin therapy. Among kidney function parameters, only GFR estimated by CKD-EPI was associated with elevated Hs-TnT. In the resulting final model, the area under the curve was 0.8001 (95%CI, 0.76-0.84) (Figure).
Final Model of Predictive Variables for High-sensitivity Troponin T
| OR (95%CI) | P | |
|---|---|---|
| Age | 1.06 (1.04-1.09) | < .01 |
| Men | 2.81 (1.67-4.73) | < .01 |
| BMI | 1.07 (1.02-1.12) | < .01 |
| CKD-EPI eGFR (mL/min/1.73 m2) | 0.96 (0.95-0.97) | < .01 |
| HF | 3.92 (1.24-12.39) | .02 |
| Insulin therapy | 1.99 (1.15-3.46) | .01 |
95%CI, 95% confidence interval; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; eGFR, estimated glomerular filtration rate; HF, heart failure; OR, odds ratio.
The TUSARC registry evaluates the presence of nonelevated and elevated Hs-TnT in a population of asymptomatic patients at very high CVR and the association of Hs-TnT elevation with a range of recorded parameters. The main findings in this registry are as follows: a) Hs-TnT was detected in all but 1 of the patients included in the registry; b) elevated Hs-TnT levels were detected in a third of the patients (32.9%); c) Hs-TnT was associated with older age, high BMI, and male sex; d) the only clinical event associated with elevated Hs-TnT was a history of clinical HF; no correlation was observed between elevated Hs-TnT and a history of coronary events or coronary revascularization; e) elevated Hs-TnT was independent of blood sugar and cholesterol levels at the time of inclusion and was also independent of microalbumin and albumin-creatine index, but was associated with lower CKD-EPI-estimated GFR, and f) insulin was the only treatment associated with elevated Hs-TnT.
Values for Hs-TnT have been recorded in several general population registries.4–6 Population registries provide real world data, without the selection bias inherent in clinical trials.
These general population registries included more patients than our population. However, comparisons are difficult because, unlike our registry, all earlier registries had a low prevalence of CVR and previous cardiovascular disease.
Nonetheless, like our registry, general population registries show an association of Hs-TnT with sex and age, with higher Hs-TnT values recorded in men and older patients.9
The differences in Hs-TnT detection between registries appear to be explained by differences in CVR. For example, all patients included in the Cardiovascular Health Study were older than 65 years, and 18% of them had a history of cardiovascular disease.5 This registry thus had higher values for mean patient age and cardiovascular history than the other population registries,4,6 reflected in the detection of Hs-TnT in 66.2% of patients.
The influence of CVR would explain not only the detection of Hs-TnT in all but 1 patient in our population, but also the high percentage of patients with elevated Hs-TnT (32.9%). While avoiding the selection bias associated with clinical trials, our results are similar to those found in clinical trials in which the inclusion criteria include ischemic heart disease or HF, in which Hs-TnT is detected in almost all patients and is elevated in 11% to 50%.2,3,9
One of the most notable findings in our study is the lack of association between Hs-TnT and a history of ischemic heart disease, manifested as angina, AMI, or revascularization, despite the high percentage of patients in our registry with a history of angina or AMI (54%). Although this finding could at first seem surprising, it is consistent with previously published series. For example, in the Dallas Heart Study,4 although the incidence of previous coronary disease was very low (3.3%), a large majority of those patients who did have coronary disease (83%) had Hs-TnT below 14 ng/L. Similarly, in clinical trials that used ischemic heart disease as an inclusion criterion, the fraction of patients with elevated Hs-TnT did not exceed 40%.2,10 This finding reinforces the idea that factors other than previous ischemic heart disease should be considered as possible causes of elevated Hs-TnT in aysmptomatic patients at high CVR.
In this regard, another important finding in our study is the association between HS-TnT and previous clinical HF events. Elevated Hs-TnT was found in almost all patients with HF associated with ventricular dysfunction, whether acute11 or chronic.3,12 In our population, 70% of patients with a clinical history of HF had elevated Hs-TnT. The many mechanisms involved in TnT release in HF patients are not limited to the presence of ischemic heart disease, and include injury mediated by inflammatory cytokines, oxidative stress, and various mechanisms triggered by wall stress, for example cellular apoptosis.13 Although Hs-TnT could be elevated by episodes of acute decompensation, our study population had no clinical events in the 3 months preceding inclusion, which should be sufficient time for TnT levels to return to baseline. The chronic Hs-TnT elevation in these patients might reflect changes to cardiac anatomy, muscular architecture, or function that are maintained beyond the release associated with acute events.
In this regard, elevated Hs-TnT has been linked to the presence of LVH measured by cardiac magnetic resonance in healthy populations, indicating troponin as a marker of structural heart disease.4 In our study, LVH showed no correlation with Hs-TnT in either the multivariable analysis or the univariable analysis. The apparent discrepancy with the cited findings may reflect the lower sensitivity of electrocardiography compared with cardiac magnetic resonance for the detection of LVH. In the low-risk ARIC registry population.6 Left ventricular hypertrophy was measured according to the Cornell criteria, as in our study, and showed an association with elevated Hs-TnT. In our high-risk population, the lack of association between elevated Hs-TnT and LVH in the multivariable analysis is probably due to the association of Hs-TnT with other factors that are better predictors than LVH.
Kidney disease is known to contribute to Hs-TnT elevation. The cardiac origin of troponin detected in kidney disease patients has been clearly established, and its elevation appears to be due to a higher prevalence of structural heart disease or ischemic heart disease, rather than to impaired clearance.14,15 In our study population, the univariable analysis showed significant associations between elevated Hs-TnT and alterations in microalbumin, albumin-creatine index, and CKD-EPI-estimated GFR. However, only GFR was associated with elevated Hs-TnT in the multivariable analysis. Although the analysis shows that Hs-TnT elevation is more likely with a lower CKD-EPI-estimated GFR, it is notable that half of the patients with elevated Hs-TnT had a GFR > 60 mL/min/1.73 m2. Many of the high CVR patients attending outpatient clinics had a slightly depressed GFR (chronic kidney disease stage 2: CKD-EPI estimated GFR ≥ 60 and ≤ 89 mL/min/1.73 m2). Elevated Hs-TnT is now an established prognostic marker of CVR in patients with advanced chronic kidney disease or on dialysis.16 Similarly, there is also a well established increased risk of cardiovascular mortality and renal failure even at early disease stages.17 Based on our findings, it would interesting to investigate whether elevated Hs-TnT in patients with high CVR and mildly depressed GFR might identify a patient subgroup with subclinical cardiac involvement and a higher risk of future cardiovascular disease. Indeed, the cardiorenal syndrome demonstrates the close interelationship between the heart and kidney in patients with acute or chronic failure of either organ.18 Thus Hs-TnT could be an early marker of negative interaction between dysfunctions in these 2 organs.
In our study, the 2 population groups showed no differences in the various CVR factors, and both groups had good lipid control and acceptable glycemic control at the time of inclusion. Despite the similar metabolic control on inclusion, the cross-sectional study design cannot exclude the possibility that control had differed between the groups before inclusion, a possibility that also holds for the development of diabetes and hypertension.
The 2 groups also showed no differences in pharmacological therapy except for treatment with insulin. Insulin therapy is given to patients with a longer history of diabetes and failed control with other pharmacological treatments. The higher rate of insulin therapy among patients with elevated Hs-TnT could reflect a longer history of the disease and worse previous metabolic control, associated with a more extensive injury to the heart muscle (eg, hypertrophy, fibrosis, microvascular dysfunction, autonomic neuropathy). The recently published BARI study is a clinical trial with defined inclusion criteria (coronary type 2 diabetes and stable ischemic heart disease with mild or no angina), and thus is not directly comparable with our registry study; however, it is interesting that the BARI study population shows an association of elevated Hs-TnT with insulin therapy and diabetes duration.10
Given the high prevalence of diabetes mellitus in our study population, diabetic cardiomyopathy19 is a possible explanation for the detected association of Hs-TnT with HF and insulin therapy. Diabetes patients develop not only epicardial coronary disease, but also microvascular disease triggered by oxidative stress, with the resulting altered vascular morphology leading to ischemia, fibrosis, and muscular rigidity.20 Unfortunately, microvascular disease can only be detected with invasive methods or complex imaging techniques.
LimitationsThis study has several limitations: a) not all patients underwent an imaging study that would permit more precise diagnosis of LVH and provide information about systolic and diastolic cardiac function; however, electrocardiographic LVH diagnosis is highly specific; b) although none of the patients included in the registry had symptoms consistent with HF, we did not measure N-terminal probrain natriuretic peptide, which might have identified incipient disease in asymptomatic patients; c) due to its cross-sectional design, the study does not provide information about prior control of risk factors or their duration before inclusion; d) the study was conducted in a single center; however, the inclusion of patients attending internal medicine and cardiology consultations permitted a more representative sampling of patients with different risk factors and cardiovascular diseases, and e) finally, the study population was not screened for silent myocardial ischemia, despite the high prevalence of diabetes mellitus in this population.
CONCLUSIONSHigh-sensitivity troponin T was detected in almost all high CVR patients receiving stable treatment in the 3 months preceding inclusion. A clinical history of HF was significantly associated with elevated Hs-TnT. High-sensitivity troponin T showed no association with a history of AMI, angina, or coronary revascularization. Patients with elevated Hs-TnT had a signficantly diminished CKD-EPI-estimated GFR, although more than half of them had a GFR > 60 mL/min/1.73 m2. Among the pharmacological treatments examined, only insulin therapy showed an association with Hs-TnT elevation.
FUNDINGThis study was funded by Roche Diagnostics.
CONFLICTS OF INTERESTNone declared.
- –
The level of Hs-TnT and the prevalence of Hs-TnT elevation have been studied in “healthy” populations (with few cardiovascular risk factors and little prior cardiovascular disease) and in clinical trial populations recruited according to strict inclusion criteria.
- –
There is a lack of population registries including patients at very high cardiovascular risk, especially in Spain.
- –
The results of previous registries and trials cannot be extrapolated to a high-risk population, leaving uncertainty about what proportion of high-risk patients have elevated Hs-TnT and the factors associated with this elevation.
- –
The present study evaluated Hs-TnT and the prevalence of elevated Hs-TnT in an aymptomatic Spanish population at very high cardiovascular risk. Elevated Hs-TnT was associated with a history of heart failure and insulin therapy, but not with a history of infarction, angina, or coronary revascularization.
- –
The results suggest several possibilities related to Hs-TnT: a) It may be an early marker of cardiorenal syndrome. b) It may be a marker of myocardial injury in the asymptomatic population with diabetes. c) It may be a marker of structural heart disease. d) It might be associated with a worse prognosis during follow-up.