Keywords
INTRODUCTION
The morphology and polarity of the P wave during atrial tachycardia (AT) in the 12-lead electrocardiogram (ECG) are useful in the identification of its place of origin.1-7 Left focal ATs frequently originate in the pulmonary veins (PV) and typically have more rapid atrial frequencies than other ATs.1 Selective stimulation of PVs and PV-AT which occur following the ablation of pulmonary veins produces characteristics that allow the exact identification of the responsible PV.2,3 However, the surface ECG has limitations in clearly establishing the PV origin of AT.4-7. In addition, it is not always possible to assess the morphology and polarity of the P wave during AT, frequently due to overlapping with the T wave on the preceding beat.7,8 The identification of a PV origin of ATs allows specific preparation (including the predicted need for transseptal puncture) of the ablation procedure in this type of tachycardia, with the added risk of systemic embolism associated with this treatment.9
Patients with paroxysmal atrial fibrillation (PAF) and sinus node disease frequently have an increased inter- and intra-atrial conduction time.10,11 The non-homogeneous propagation of the sinus impulses results in morphological abnormalities in the sinus P wave, with an increase in its duration and dispersion. The presence of notches and biphasic P waves, especially in leads II-III and a VF of the ECG, are a result of this process.12,13
In this study the differential clinical and electrophysiological characteristics associated with PV-AT are analysed, including the presence of morphological abnormalities in the sinus P wave, compared with other AT origins.
METHODS
Study Population
Those consecutive patients who underwent mapping and ablation of focal AT refractory to antiarrhythmic drugs, with no prior ablation of the pulmonary veins were considered for inclusion in the study. The selection of patients was made during outpatient consultations in the arrhythmia and electrophysiology unit, where the diagnosis of refractory atrial tachycardia was made, with an indication of electrophysiological study and/or radiofrequency ablation. The consecutive patients who underwent ablation of atrial tachycardia were brought together at Hospital de la Santa Creu i Sant Pau and Hospital del Mar, in Barcelona, in collaboration with Hospital of the University of Pennsylvania, Philadelphia (PA). Prior to the study, all patients received blood tests and 2D echocardiography (with measurement of the antero-posterior diameter of the left atrium using M mode, parasternal view, long axis). The risks of the procedure were discussed in detail, and all patients gave their informed consent in accordance with the guidelines of each institution. From January 2003 until June 2006, 87 patients who underwent ablation of 95 ATs were included. For comparative analysis, they were grouped into patients with PV-AT (group 1, n=25/26 AT), PV-AT with associated recorded PAF (group 2, n=18/18 AT), other left ATs (group 3, n=7/7 AT) and right ATs (group 4, n=37/44 AT). A group was defined with isolated PV-AT with no associated PAF (group 1), to exclude PAF itself as a potential confounding factor in the results obtained.
Protocol of Study and Ablation
All antiarrhythmic drugs were discontinued prior to the procedure for at least 5 half lives, and no patient was under treatment with amiodarone.
During the electrophysiological study, reproducible tachycardia induced by atrial stimulation was considered the clinical AT when clinical symptoms were mimicked in the absence of any other inducible tachycardia that could account for them. Macroreentrant arrhythmias (flutters) were excluded from our analyses. This mechanism is suspected in the presence of electrical activity which covered practically the entire cycle of tachycardia in the studied cardiac chamber and without an isoelectric line in the surface ECG. The identification of an obstacle or a functional or anatomical electrical barrier and the identification of an isthmus or "protected" area of the tachycardia circuit supported the microreentrant diagnosis. Finally, the recording of return cycles similar to those of tachycardia in distant atrial regions, in addition to the criteria of constant and progressive fusion (infrequent in macroreentrant tachycardias) were also suggestive of a macroreentrant mechanism.14,15 Abnormal automatism or triggered activity were considered as an arrhythmogenic mechanism if they satisfied at least 2 of the following 3 criteria16: a) prolonged or "incessant" episodes of arrhythmia, with the on and off phenomenon and "warm-up" at the beginning or "cool-down" at the end of the AT; b) Inability to reproducibly initiate or terminate the tachycardia with programmed atrial stimulation; and c) the need to use burst pacing or isoproterenol infusion to induce the tachycardia. When these criteria were not satisfied, and with the macroreentrant mechanism having been excluded, a microreentrant mechanism of the arrhythmia was suspected via entrainment techniques during tachycardia.15
The origin of the AT was identified in an electroanatomical activation map (CARTO®, Biosense Webster, Diamond Bar, CA, USA), based on the termination of the tachycardia during ablation and/or inability to reinduce the arrhythmia with programmed atrial stimulation, burst pacing or isoproterenol infusion following ablation and during a subsequent observation period of 30 minutes. Special care was taken to correlate relate the efficacy of the procedure with the application of radiofrequency and not with a possible mechanical blockage of the tachycardia during its study. Ablation was carried out by means of non-irrigated tip catheters: a 4 mm catheter with right ATs and an 8 mm catheter with left ATs (NAVISTAR®, Biosense Webster, Diamond Bar, CA, USA) using energies of 30-50 W and temperatures of 55ºC. In the case of AT-PV, circumferential ablation was carried out, with the aim of electrical isolation of the vein. The cycle length of the AT was measured digitally using electronic calipers during the electrophysiological study with intracavitary signals at a speed of 100 mm/s. The 12-lead ECG was analysed offline using the Prucka® Cardiolab recording system (Houston, TX, USA) and Lab Pro (C.R. Bard Inc., New Jersey, USA) polygraphs, with a bandwidth of 0.05-100 Hz. The maximum sinus P wave duration was measured at the beginning of the procedure (prior to ablation, in order to avoid morphological changes due to the actual application of radiofrequency) in each ECG lead; any notches present in two or more ECG leads were also measured, with a peak-peak distance of the notched P wave ≥1 mm (0.04 seconds).11,17,18
Statistical Analysis
Variables are described as the mean (standard deviation). The continuous variables were compared by means of the c2 test. The continuous variables were analysed by means of analysis of variance (ANOVA). A value of P<.05 was considered statistically significant.
RESULTS
Study Population
The demographic characteristics of the study population (average age, 52 [15] years, 46 women) and the distribution of origin of the ATs are shown in Table 1 and Figure 1. Patients with isolated ATPV (group 1) were younger than the rest (P<.05); however, no differences in gender were observed between groups. Groups 1 (PV-AT) and 4 (right AT) had a significantly lower incidence of structural heart disease and atrial dilatation (P<.05 and P<.01 respectively) compared with groups 2 and 3 (PV-AT associated with PAF and other left ATs).
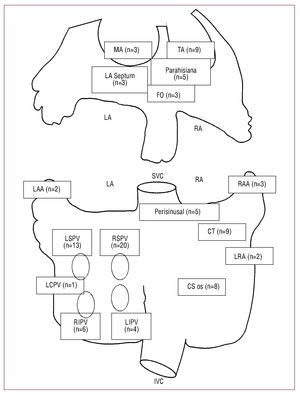
Figure 1. Distribution of the origin of atrial tachycardia in the study population. Diagram of the right and left atria indicating origin of arrhythmia: CT, crista terminalis; CS os, coronary sinus ostium; FO, fossa ovalis; IVC, inferior vena cava; LA, left atrium; LAA, left atrial appendage; LCPV, left common pulmonary vein; LIPV, left inferior pulmonary vein; LRA, lateral right atrium; LSPV, left superior pulmonary vein; MA, mitral annulus; RA, right atrium; RAA, right atrial appendage; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SVC, superior vena cava; TA, tricuspid annulus.
Pulmonary Vein-Atrial Tachycardia
PV-AT associated or not with PAF (groups 1 and 2), originated mainly from the right superior and left superior pulmonary veins (Table 1). Furthermore, PV-ATs had shorter cycle lengths (faster heart rates) than other left or right ATs (groups 3 and 4): 289 (45) ms (group 1) and 280 (48) ms (group 2) versus 392 (106) ms (group 3) and 407 (87) ms (group 4); P<.05. The arrhythmogenic mechanism was almost uniformly abnormal automatism or triggered activity in PV-ATs (in 24/25 in group 1 and 17/18 in group 2). On the other hand, re-entry was associated more frequently with groups 3 (4/7) and 4 (16/44); P<.05 (Table 2).
Sinus P Wave: Morphological Abnormalities
Patients with left ATs (groups 1 to 3) were differentiated by the presence of a more prolonged sinus P wave than those in group 4 (right ATs): 126 (25) ms (group 1), 133 (23) ms (group 2) and 136 (37) ms (group 3) versus 106 (20) ms (group 4); P<.01. The proportion of patients with a notch in the sinus P wave was equally high in groups with left ATs: 15/25 (60%, group 1), 11/18 (61%, group 2), and 5/7 (71%, group 3) versus 10/37 (27%, group 4); P<.025.
Bearing in mind the younger age of the group of patients with isolated PV-AT, the diagnostic value of the prolongation and notch of the sinus P wave was specifically analysed in patients below the age of 50 years with no structural heart disease (n=37). A duration ≥110 ms of the sinus P wave (sensitivity 68%, specificity 69%, positive predictive value 72%, negative predictive value 65%; P=.04) and the presence of a notch on the sinus P wave (sensitivity 79%, specificity 70%, positive predictive value 61%, negative predictive value 84%; P=.02) were indicators of pulmonary vein origin of the AT in this subgroup (Figures 2 and 3).
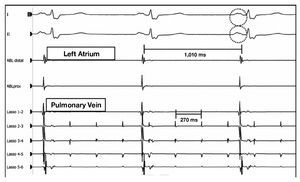
Figure 2. Atrial tachycardia of the left superior pulmonary vein. Effective ablation following isolation of the vein. During the application of radiofrequency, it can be observed in the recordings that the tachycardia remains within the pulmonary vein ("Lasso" catheter) while the atrium is in sinus rhythm (ablation catheter). The presence of a notch on the P wave of the represented ECG leads is noteworthy. Distal ABL indicates ablation catheter, distal pole; proximal ABL, ablation catheter, proximal pole.
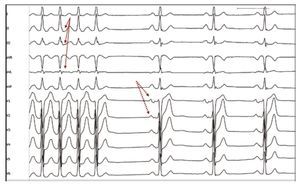
Figure 3. Identification of abnormalities of the sinus P wave in a patient with AT-PV. ECG reading of an atrial tachycardia of the left superior pulmonary vein interrupted during application of radiofrequency. The morphology of the P wave during tachycardia could not be observed due to its overlapping with the preceding T wave (arrows). In sinus rhythm the notched, prolonged P wave, characteristic of these patients (dotted arrows), which was already present prior to the application of radiofrequency, can be easily observed.
Results of the Ablation
Post-ablation, a clinical follow-up was carried out for 24 (14) months. In patients with isolated PV-AT (group 1), the follow-up period was 34 (10) months. Acute success of the procedure was achieved in 24/26 cases of group 1, with 2 clinical recurrences in the follow-up period. During the follow-up period, PAF was observed in only one patient from this group. In group 2, acute efficacy was obtained in 17/18 procedures, with 4 recurrences and relapse of PAF in 6. Ablation of left ATs (group 3) was effective in 6/7 patients, with one recurrence. Finally, ablation of right ATs was effective in 39/44 patients, with 5 additional recurrences in the follow-up period.
DISCUSSION
This study demonstrates that when a clinical suspicion of atrial tachycardia is established in a patient below the age of 50 years, with no cardiopathy or atrial dilatation, the presence of prolongation (≥110 ms) and a notch on the sinus P wave is suggestive of an origin in the pulmonary vein (sensitivity 68%-79% and specificity 69%-70%), with the resulting potential need for transseptal puncture for the ablation of the responsible PV. In older patients, the presence of cardiopathy or association of PAF with these morphological abnormalities of the sinus P wave are not as specific. Furthermore, a rapid atrial frequency (higher than 200 per minute: atrial cycles of 289 (45) ms and 280 (48) ms in groups 1 and 2) is characteristically associated with PV-AT and not with other left or right ATs.
This study confirms that PV-ATs are caused predominantly by non-reentrant arrhythmogenic foci (abnormal automatism/triggered activity) if there is no previous history of PV ablation.19 PV-AT is considered an independent entity and does not necessarily precede paroxysmal atrial fibrillation, despite sharing a common predominant origin in the upper pulmonary veins.20 Our study recorded one single episode of PAF in our population with isolated AT-PV during a post-ablation follow-up period of 34 (10) months, although the design of the study and the influence of a circumferential ablation of the responsible PV make it impossible to assess the etiopathogenic relationship between PV-AT and PAF.
The abnormalities of the sinus P wave observed in patients with PV-AT are a sign of an increase in the inter- and intra-atrial conduction time of the sinus impulses. Experimental models of rapid atrial stimulation reproduce electrical remodelling which gives rise to an increase in the duration and dispersion in the conduction of the sinus impulse. PVAT, a particularly rapid form of atrial tachycardia, may induce the morphological changes identified in the sinus P wave via this mechanism, although additional scientific evidence is required to confirm this.21 This increase in the inter- and intra-atrial conduction time has been strongly linked to PAF, especially in the presence of concomitant sinus node dysfunction.10,11 Finally, the electrical activity within the pulmonary vein (through sleeves) may prolong the duration of the mid and distal sections of the sinus P wave, which is also demonstrated reducing the duration of the sinus P wave following isolation of pulmonary veins for the treatment of atrial fibrillation.22-24 These abnormalities of the sinus P wave are less pronounced in right ATs. The lower atrial frequencies of these ATs may have favoured a lower degree of atrial "remodelling." Finally, the presence of underlying occult myocardial disease (myocarditis, necrosis, and myocardial fibrosis), more related with the origin of the "isolated" atrial fibrillation, may be involved in the etiopathogenesis of the prolongation/notch of sinus P and of PV-AT itself.25
In this study we also observed that patients with PV-AT associated with PAF are older aad have larger atria compared with patients with isolated PVAT. The possibility that the atrial 'remodelling' in these patients is an evolutive process leading to atrial dilatation and degeneration and to atrial fibrillation after a critical time is not demonstrated.
Limitations
An isoproterenol infusion was occasionally necessary to induce PV-AT, and the AT cycle may have been influenced by this. However, previous data confirm that PV-ATs are more rapid than other ATs.
This study does not specifically analyse the interobserver variability in the acknowledgement and measurement of the abnormalities of P wave morphology in patients with AT. In addition to this, we cannot discoumit that the use of antiarrhythmic drugs may contribute to these abnormalities of the sinus P wave, despite being interrupted 5 half lives prior to the procedure, and no patient having been on amiodarone.
In this study, the influence of a higher or lower origin in the right atrium of the sinus impulse on the inter- and intra-atrial conduction times and, consequently, on the duration and presence of a notch on the sinus P wave, was not analysed. Finally, the number of patients with left ATs (group 3), despite being statistically sufficient, may not be representative of this subgroup of patients with left ATs not starting at the PV.
CONCLUSIONS
The cases of PV-AT are almost uniformly due to a mechanism of abnormal automatism/triggered activity and have more rapid frequencies than other ATs. Identification of prolongation ≥110 ms) and of a notch on the sinus P wave, especially among young patients (<50 years) without baseline heart disease, is a sensitive and specific indicator of AT originating in the PV. These findings have significant implications for the selection of AT ablation strategy.
ABBREVIATIONS
AT: atrial tachycardia
ECG: electrocardiogram
PAF: paroxysmal atrial fibrillation
PV: pulmonary vein
SEE ARTICLE ON PAGES 134-6
Correspondence: Dr. V. Bazán.
Unidad de Electrofisiología. Servicio de Cardiología. Hospital del Mar, IMAS, UAB.
Pg. Marítim, 25-29. 08003 Barcelona. Spain.
E-mail: vbazan@imas.imim.es
Received October 16, 2008.
Accepted for publication July 6, 2009.