Keywords
INTRODUCTION
Aortic valve stenosis is the most common valve lesion in Europe and the prognosis using medical treatment is very poor.1 Surgical replacement of the aortic valve is the treatment of choice in patients with severe aortic stenosis. In the majority of patients the relief of symptoms and an increase in survival is achieved by this procedure. However, owing to the fact that the most common aetiology for aortic stenosis in Western countries is degenerative, the patients are normally elderly (in adults aged ≥75 years, aortic stenosis is present in 4.6% of cases),2 so that they often have concomitant pathologies which increase surgical risk and post-operative morbidity. In high-risk patients with left ventricle dysfunction, concomitant coronary disease, previous coronary revascularization surgery, chronic obstructive pulmonary disease and/or who are of advanced age the mortality rates for surgical aortic valve replacement increases significantly.2-4 In fact, according to Euro Heart Survey data, up to a third of patients with aortic stenosis were rejected for surgery because they presented excessive associated co-morbidities or a short life expectancy.5 This is why aortic valve prostheses have been designed, since they can be implanted by means of catheters and currently constitute a therapeutic alternative for high operative risk patients with severe aortic stenosis who have been rejected for surgery.6-12
The aim of this study is to present the initial experience in 3 Spanish centres of the percutaneous implantation of the CoreValve® self expanding aortic valve prosthesis. We have analyzed the hospital results and follow-up in the medium term.
METHODS
Design
Multi-centre prospective study.
Study Population
After the evaluation of each case by a multidisciplinary team (clinical cardiologists, cardiac interventionists, and surgeons), a total of 108 patients (43 in Malaga, 34 in Cordoba, and 31 in Asturias) suffering from severe symptomatic aortic stenosis which posed high surgical risk (n=44) or who rejected surgical intervention (n=64) were included in the study. To assess the suitability of patients the following examinations were performed before the procedure: coronariography, aortography with injections in the iliofemoral region, transthoracic and/or transoesophageal echocardiogram (if the transthoracic examination proved inconclusive), and in some cases CT with the injection of a contrast agent. Operative risk was calculated using the logistic EuroSCORE.13
Inclusion criteria: patients with severe symptomatic aortic stenosis affecting an area <1 cm2; diameter of the aortic ring measured by transthoracic and/or transoesophageal echocardiogram ≥20 mm and ≤27 mm; diameter of the ascending aorta at the level of the sinotubular junction ≤40 (small prosthesis) or ≤43 mm (large prosthesis). Exclusion criteria: hypersensitivity or contraindication to the administration of any of the medications needed during the procedure; myocardial infarction during the 30 days prior to the procedure; coronary angioplasty during the 15 days preceding the procedure or scheduled during the month following the procedure; presence of thrombi in left cavities; ejection fraction <20%; recent stroke; sepsis or endocarditis; aneurisms of the aorta; coagulopathy or haemorrhagic diathesis; and severe mitral regurgitation with inversion of pulmonary vein flow.
Description of the Device
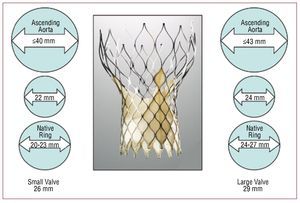
The device (CoreValve ReValving System®) consists of 3 elements: a) triple-valve aortic prosthesis made of pig pericardium, which is fitted on top of a self expanding nitinol stent—there are 2 valve sizes (Figure 1), one for aortic rings from 20 to 23mm in diameter (small prosthesis, the portion inserted into the native ring measuring 26 mm) and another for 24 to 27 mm rings (large prosthesis, the portion inserted in the native ring measuring 29 mm)—; b) 18 F releasing catheter; and c) charging device.
Figure 1. Characteristics of the 2 Corevalve® valves which are available: small valve, measuring 26 mm in the portion which is inserted into the ring of the patient, for ascending aortas ≤40 mm and rings from 20-23 mm, and large valve, measuring 29 mm in the portion which is inserted into the ring of the patient, for ascending aortas ≤43 mm and rings ranging from 24-27 mm.
Procedure
Taking into account the fact that the training period required for learning to perform valve implants (all the procedures were supervised by professionals) and that initially the availability of the professionals who supervised the procedures was very limited, aortic valvuloplasty was performed on 7 patients awaiting the valve implant who were in a critical state.
In all cases the valves were implanted in the haemodynamics laboratory under a general anaesthetic (58 patients) or a local anaesthetic combined with deep sedation (50 patients). Cephalosporins were used for antibiotic prophylaxis or vancomicine in cases of allergy to betalactamics.
Vascular access was femoral, the procedure being entirely percutaneous in the vast majority of cases and in a minority subclavian (4 left and 1 right access), which involved open surgery (in cases of excessive calcification, twisted arteries or atherosclerosis of the iliofemoral region, or when the diameter of the iliac/femoral arteries was <6 mm).
After implanting a pacemaker catheter via the right jugular vein, the femoral artery selected for valve implantation was punctured and a vascular closure device (PROSTAR XL®) was fitted. Puncture was performed using fluoroscopy, injecting a contrast agent through a catheter inserted into the contralateral femoral artery.
After that, in the cases in which an aortic valvuloplasty had not been performed within the month prior to the procedure (101 patients), aortic valvuloplasty was performed, employing simultaneous hyperstimulation at a frequency of 180 bpm to avoid displacement of the balloon. Twenty-two mm (small valve implantation cases) or 25 mm (large valve implantation cases) balloons were used. Then the device was released retrogressively, guided by fluoroscopic and aortographic imaging. After implantation the gradient between the left ventricle and the aorta was measured and the presence of residual aortic regurgitation was evaluated. In cases of aortic regurgitation of an angiographic degree >2, postdilation and/or traction of the valve was performed. The procedure was completed by percutaneous closure of both femoral arteries. The femoral artery through which the device was implanted was closed using the previously implanted PROSTAR XL® and the contralateral femoral artery was closed using PERCLOSE® or ANGIOSEAL®. When access was subclavian, the artery was surgically exposed and then punctured using the Seldinger technique, and the procedure was identical to that used for femoral access. Finally, the artery was surgically closed.
Anti-platelet and Anti-thrombotic Medication
Where there were no contraindications, all the patients were administered 100 mg of aspirin before the procedure and indefinitely thereafter. In addition, patients received a loading dose of 300 mg of clopidogrel (administered a few days before the procedure) and subsequently 75 mg over a period of at least 3 months. During the procedure sodium heparin was administered, adjusting the dose for weight (80-100 U/Kg).
Post-procedure Care Measures
After the procedure the patients remained in hospital and were continuously monitored in a critical care unit for 48 hours, when they were transferred to the ward and the transitory pacemaker was removed. If any AV (atrial-ventricular) block episodes were recorded during this period, a definitive pacemaker was implanted.
Follow-up
All the patients were followed up 30 days later and every 6 months thereafter. The average period for follow-up was 7.6 months.
Definitions
The following definitions apply: success of the procedure means the correct implantation and normal functioning of the prosthesis (evaluated by angiography and echocardiogram), in the absence of mortality during the procedure. Vascular complications: aortic dissection, failure of the percutaneous closure device, iliac or femoral rupture, haemorrhage requiring surgery, and or red blood cell transfusion. Mortality at 1 month: death due to any cause which occurred in the hospital or during the month following the procedure. Mortality after the first month: death due to any cause which occurred after the first month following the procedure. Total mortality: this is the sum of both mortality figures.
Statistical analysis
The data is expressed as the mean (standard deviation) in the case of continuous variables and as a number (percentage) in the case of categorical variables. A basic descriptive analysis and Kaplan-Meier survival analysis were performed. The data was analyzed using the SPSS version 16 statistical program (Chicago, Illinois, USA).
RESULTS
Characteristics of the Population
From December 2007 to July 2009, 108 patients (49 men; average age, 78.6 years; range, 50-92) were included in the study. The baseline characteristics of the population are shown in Table 1. All the patients had severe symptomatic aortic valve stenosis with an echocardiographically measured transaortic systolic gradient of 83.8 mm Hg (range, 34-163) and a mean of 55 mm Hg (range, 20-93). The average aortic valve area, calculated by echocardiogram prior to the procedure was 0.63 (0.2) cm2. The average logistic EuroScore was 16% (13.9%) (range, 2.27%-86.4%), with 21.3% of patients scoring ≥20%, and 58.4% of the patients were in NYHA functional class III or IV.
Procedure Data
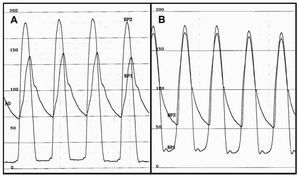
Aortic valvuloplasty was performed prior to the procedure in 7 patients (6.5%). In 95.4% of cases vascular access was via the femoral artery. In 64 cases (59.3%) a small valve was implanted. The procedure was completed successfully in 106 cases (98.1%, Table 2). In 1 of the 2 failed cases, a second valve had to be implanted on top of the first one during the same procedure, owing to the fact that the first one was implanted too low and severe aortic regurgitation persisted, a good result being obtained after implantation of the second valve. In the other case, the aortic ring ruptured following valvuloplasty. Postdilation of the valve was performed in 25 cases (23.1%). None of the patients presented residual angiographic aortic regurgitation above grade 2. The haemodynamic peak-to-peak gradient after the procedure was 2.4 (4.2) mm Hg (Figure 2).
Figure 2. Intraventricular and intra-aortic pressure gradients before (A) and after (B) implantation of the aortic prosthesis. In panel B a minimum residual aortic gradient can be seen.
The maximum instantaneous transaortic gradient measured by echocardiogram was 12.6 (6) mm Hg. Percutaneous vascular access closure was successful in 104 cases (96.3%).
Complications of the Procedure
The complications derived from the procedure are shown in Table 2. Vascular complications occurred in 6 patients (5.6%), 2 of whom had a logistic EuroScore >20%. Three patients needed urgent operations, 2 due to failure of the percutaneous closure device and another to iliac rupture, and one of them, an immunodepressed patient with primary agammaglobulinaemia, died in a state of septic shock. One patient presented a femoral pseudoaneurysm, which remitted following the injection of thrombin and local compression. In 1 case there was a dissection of the ascending aorta at the level of the valsalva sinus caused by the dilator of a large valve sheath, which remitted when the valve was implanted. Another patient developed a substantial haematoma with anaemization, which required blood transfusion. As far as non-vascular complications are concerned, 3 patients developed cardiac tamponade, 1 due to perforation of the rigid preformed guide tube, another to perforation of the transitory pacemaker implanted during the procedure and the third as a result of rupture of the aortic ring after performing the aortic valvuloplasty. A patient who had undergone previous coronary revascularization with a permeable bridge between the left internal mammary artery and the anterior descending artery suffered a myocardial infarction, owing to dissection of the mammary artery during the procedure (left subclavian access), but was treated successfully by implanting a stent in the artery. In another case, when the aortic valve was implanted, dynamic obstruction to the blood flow in the left ventricle outflow tract developed as a result of anterior systolic movement of the mitral valve, accompanied by severe mitral regurgitation, which remitted when treated with intravenous beta blockers. Owing to the presence of AV blocks, a definitive pacemaker had to be implanted in 38 patients (35.2%).
Follow-up at 1 Month
During the hospitalization period 8 patients died (7.4%) (Table 3). The average logistic EuroScore of these patients was 23 (26.6) (range, 7-86.4). Two of the 8 patients who died had a logistic EuroScore >20%. Three patients died as a result of residual moderate-severe aortic regurgitation. In 2 of them, non-urgent cardiac surgery was carried out, with the result that both patients died, one in a state of cardiogenic shock 11 days after the procedure and another from a massive retroperitoneal haemorrhage 10 days after surgery. The causes of death in the other 5 patients who died were: perforation of the left ventricle by the rigid preformed guide tube, pericardial tamponade following valvuloplasty, cardiogenic shock in a patient with severe diastolic dysfunction and a normal valve, septic shock in an immunodepressed patient, and the impossibility of extubation in an elderly patient.
Follow-up After the First Month
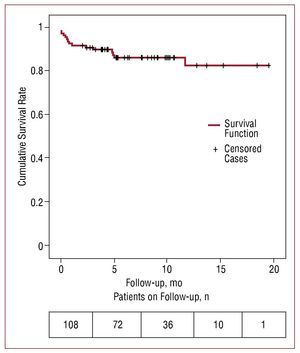
After the first month of follow-up 7 patients died (6.5%) (Table 3), with a Euroscore of 12.6% (4.9%) (range, 6.8-18.6). After the first month of follow-up 6 patients died as a result of non-cardiological causes (multi-organ failure after radiotherapy used to treat spinocellular carcinoma, hypercapnic encephalopathy in a patient with severe chronic obstructive pulmonary disease, hypercapnic encephalopathy in a patient with pulmonary fibrosis, pulmonary thromboembolism, stroke, acute pancreatitis) and another died suddenly 5 months after the procedure. Overall survival after 1 year estimated using the Kaplan Meier method was 82.3% (Figure 3). The average follow-up period was 7.6 months.
Figure 3. Estimation of survival at one year for the study population (n=108) by means of Kaplan Meier survival analysis.
DISCUSSION
The results of our study confirm previously published data which demonstrates that it is possible to perform implantation of the CoreValve® percutaneous aortic valve with a high success rate in patients with severe aortic stenosis.7 In 1 patient, a second valve had to be implanted immediately, due to low implantation associated with severe aortic regurgitation, and a good final result was achieved. In another patient, rupture of the aortic ring occurred following valvuloplasty. This implantation success rate (106/108; 98.1%) is higher than the success rate reported by Grube et al (88%)7 and similar to that of Piazza et al (97%).14
Hospital mortality was 7.4%, lower than the result obtained applying the algorithm of the EuroSCORE (average, 16%).13,15 The comparison of the mortality rate with the EuroSCORE must be made with caution, given that there are studies which demonstrate that this predictive model may overestimate the mortality of these patients.16-19
Despite these encouraging results, our attention is drawn to the high rate of acute complications derived from the procedure as a result of cardiac tamponade and vascular complications. With regard to the prevention of cardiac tamponade, we consider the correct preshaping of the rigid guide tube to be a very important factor, as well as the continual observation of its distal edge using fluoroscopic control to rapidly detect any displacements of the guide tube and the associated risk of perforation. The use of low-calibre endocavitary pacemakers (4 Fr) and their correct and careful positioning is also very important. Vascular complications are associated with high mortality, so the careful manipulation of the device and the correct selection of patients is extremely important to avoid arterial rupture and bleeding. For all these reasons we believe that post-procedure care in a critical care unit is very important to monitor patients closely and to quickly detect and treat complications derived from the procedure.
The pacemaker implantation rate for our series (35.2%) concords with the latest data published by Grube et al20 and is high in comparison with the first series published.14 In 136 patients treated with the 3 generations of the CoreValve®, a definitive pacemaker was implanted in 33.3% of the patients treated with the third-generation valve20; however, in 646 patients with aortic stenosis who had a CoreValve® implanted, the percentage of definitive pacemakers implanted was 9.3%.14 Both percentages are higher than those published in surgical series, which are around 6%-6.5%.21-23 The differences found between both studies with regard to the pacemaker implantation rate is explained by the fact that different policies were followed with respect to the definitive pacemaker implantation criteria. This high implantation rate may be due to the fact that many indications are prophylactic, given that we are faced with a new type of patient in whom we do not know how certain electrocardiographic disorders which have recently appeared, such as bradycardias or blocks of the left branch of the aorta, will evolve. In our series a patient who developed a left branch block after implantation (QRS width of 180 ms) suddenly died during follow-up. There is a need for specific studies which investigate these aspects to establish the indications for definitive pacemaker implantation in these patients.
As for mortality 1 month after the procedure (8 patients; 7.4%), 60% of the deaths occurred in the first patients included in the study. This figure again demonstrates the importance of the learning curve for the procedure and the concentration of mortality in this period, an aspect which has already been observed by other authors.7 Webb et al9 demonstrated with the Cribier Edwards valve that the accumulation of experience increases the success rate for the procedure, as the success rate for the first 25 patients who were treated was 76%, this rate increasing to 96% in the following 25 patients. When the mortality at 30 days was compared for both groups, a significant difference between both groups of treated patients was also observed (16% vs 8%).
From the point of view of the procedure, when it is compared with the Edwards-Sapien® aortic valve, the CoreValve® prosthesis presents certain advantages.24 It is implanted (both the 26 mm and 29 mm versions) by means of an 18-Fr introducer, while the Edwards-Sapien® prosthesis is implanted using 22-Fr (the 23 mm model) and 24-Fr (the 26 mm model) introducers. This is important, given that vascular complications represent a very significant percentage of the morbidity-mortality associated with the procedure. Also, high-frequency pacemaker over-stimulation is required for the implantation of the Edwards-Sapien® valve, while this is not necessary with CoreValve® implantation.
The measurement of haemodynamic pressure and gradients following the procedure demonstrates the efficacy of this new technique. As soon as the valve has been implanted, the aortic transvalvular gradients fall considerably and this is usually accompanied by aortic regurgitation, which is generally not severe. However, the data from our study shows that it was necessary to postdilate the valve, owing to the presence of an aortic regurgitation level >2 in 17.8% of cases, a figure which is similar to that in previous series.7,14 The consequences of this postdilation and its effect on the structure of the valve in the long term will be studied in future clinical trials involving long-term follow-up.
Limitations
In this multi-centre study we analyzed the results for CoreValve® aortic valve implantation in a limited number of patients and for a short follow-up period (average follow-up period, 7.6 months), without any comparison with a control group. The survival after 1 year demonstrated in our study must be interpreted with caution, given that we have a follow-up period longer than a year in 22 patients (Figure 3). We believe that studies with a greater sample size and a follow-up period of no less than 5 years are necessary to evaluate the efficacy of the device.
CONCLUSIONS
Our initial experience suggests that percutaneous aortic valve replacement constitutes a safe and viable therapeutic option for high operative risk patients with severe aortic stenosis.
ABBREVIATIONS
AR: aortic regurgitation
AMI: acute myocardial infarction
BMI: body mass index
EuroSCORE: European System for Cardiac
Operative Risk Evaluation
NYHA: New York Heart Association Class
PCI: percutaneous coronary intervention
SEE ARTICLE ON PAGES 131-3
Correspondence: Dr. C. Morís de la Tassa.
Área del Corazón, Hospital Universitario Central de Asturias, Celestino Villamil, s/n. 33006 Oviedo, Spain.
E-mail: cesarmoris@gmail.com
Received March 25, 2008.
Accepted for publication August 7, 2009.