Recently, a new electrocardiography algorithm has shown promising results for the the diagnosis of acute myocardial infarction in the presence of left bundle branch block (LBBB). We aimed to assess these new electrocardiography rules in a cohort of patients referred for primary percutaneous coronary intervention (pPCI).
MethodsRetrospective observational cohort study that included all patients with suspected myocardial infarction and LBBB on the presenting electrocardiogram, referred for pPCI to 4 tertiary hospitals in Barcelona, Spain.
ResultsA total of 145 patients were included. Fifty four (37%) had an ST-segment elevation myocardial infarction (STEMI) equivalent. Among patients with STEMI, 25 (46%) presented in Killip class III or IV, and in-hospital mortality was 15%. Smith I and II rules performed better than Sgarbossa algorithms and showed good specificity (90% and 97%, respectively) but their sensitivity was 67% and 54%, respectively. In a strategy guided by Smith I or Smith II rules, 18 (33%) or 25 (46%) patients with STEMI would have not received a pPCI, respectively. Moreover, the severity and prognosis of STEMI patients was similar regardless of the positivity of Smith rules. Cardiac biomarkers were positive in 54% of non-STEMI patients, limiting their usefulness for initial diagnostic screening.
ConclusionsDiagnosis of STEMI in the presence of LBBB remains a challenge. Smith rules can be useful but are limited by suboptimal sensitivity. The search for new electrocardiography algorithms should be encouraged to avoid unnecessary aggressive treatments in the majority of patients, while providing timely reperfusion to a high-risk subgroup of patients.
Keywords
The presence of complete left bundle branch block (LBBB) is associated with repolarization abnormalities that hinder the diagnosis of acute myocardial infarction (AMI). Several electrocardiographic criteria have been proposed to diagnose AMI in the presence of LBBB, but none of them have achieved optimal diagnostic performance.1–5
Amid these diagnostic uncertainties, the presence of new or presumably new LBBB in association with ischemic symptoms has traditionally been considered an electrocardiogram (ECG) equivalent of ST-segment elevation myocardial infarction (STEMI) that should prompt emergent reperfusion.6–7
However, several studies have consistently reported a low incidence of AMI among patients with LBBB referred for primary percutaneous coronary intervention (pPCI).8–10 In view of this evidence, the latest guidelines of the American Heart Association11 state that, in patients with ischemic symptoms, LBBB alone is not diagnostic of STEMI.
An improvement in the the ECG diagnosis of STEMI in patients with LBBB is crucial to adopt an appropriate treatment strategy in each case. Recently, Smith et al.12 proposed 2 modifications of the Sgarbossa rules that, according to their results, significantly increase their sensitivity.
The main objective of the present study was to assess the diagnostic yeld of these new ECG algorithms in a cohort of patients with LBBB referred for pPCI.
MethodsStudy Design and Patient SelectionRetrospective observational cohort study. In October 2009, the Codi IAM network13 was started in Catalonia (Spain), with the aim of providing early reperfusion, mainly through pPCI, to any patient with a suspected STEMI presenting within the first 12hours after symptom onset. According to the network protocol, the presence of new or presumably new LBBB in association with ischemic symptoms is considered a STEMI equivalent and is an indication for pPCI.
The present study was a collaborative project conducted in 4 tertiary care hospitals in Barcelona, which are referral centres for pPCI. At each hospital, the clinical data of patients referred for pPCI are recorded prospectively in a dedicated database.
All patients who were referred for pPCI due to LBBB were retrospectively retrieved. They were included in the study if the ECG prompting the referral for pPCI (recorded when the patient was symptomatic) was available for analysis and complete LBBB was confirmed. If more than 1 pre-PCI ECG was available, all were analyzed.
The study was approved by the local ethics committee and all patients gave written informed consent.
Electrocardiographic AnalysisAll ECGs were analyzed by 2 independent cardiologists (A. Di Marco and M. Rodríguez) who were blinded to the patients’ clinical data. If there was disagreement, the evaluation of a third cardiologist (I. Anguera) was requested. Left bundle branch block was defined as the presence of QRS duration > 120 msec, QS or rS in V1, and the absence of Q wave in V6. ST-segment deviation was calculated at the J point. In line with the methods used in the report by Smith et al.,12 all measurements were to the nearest 0.5mm (0.05mV) and relative to the PR segment. ST-segment deviation was considered concordant or discordant with respect to the main QRS axis.
The 3 Sgarbossa criteria, 2 Sgarbossa algorithms, and 3 Smith algorithms were evaluated and are described in detail in Table 1. To limit the influence of a wandering baseline and interbeat ST and QRS variability, each algorithm was considered positive when present in more than 50% of the beats available in 1 lead.
Description of the Electrocardiographic Criteria and Algorithms Evaluated
| Criteria | Description |
| Sgarbossa score = 5 | Concordant ST-segment elevation ≥ 1 mm |
| Sgarbossa score = 3 | ST-segment depression ≥ 1 mm in leads V1-V3 |
| Sgarbossa score = 2 | Discordant ST-segment elevation ≥ 5 mm |
| Algorithms | Description |
| Sgarbossa score ≥ 3 | Concordant ST-segment elevation ≥ 1 mm and/or ST-segment depression ≥ 1 mm in leads V1-V3 |
| Sgarbossa score ≥ 2 | Sgarbossa score ≥ 3 and/or discordant ST-segment elevation ≥ 5 mm |
| Smith I rule (rule III in the original report) | Sgarbossa score ≥ 3 and/or discordant ST-segment elevation with a ST/S ratio ≤ -0.25 |
| Smith II rule (rule IV in the original report) | Sgarbossa score ≥ 3 and/or discordant ST-segment deviation with a ST/S or ST/R ratio ≤ −0.3 |
| Smith III rule (rule V in the original report) | Discordant ST-segment deviation with a ST/S or ST/R ratio ≤ -0.3 |
A STEMI equivalent was defined as the presence of an acute coronary occlusion (Thrombolysis In Myocardial Infarction [TIMI] 0) or an acute lesion with TIMI flow ≥ 1 associated with a significant rise in cardiac biomarkers. Coronary stenosis was considered acute when signs of thrombus or ulceration could be identified on the angiogram. Since the 4 hospitals used different methods to analyze cardiac biomarkers, values were normalized using the ratio of the peak value of the biomarker with the upper normal limit of each specific test used. A significant rise in biomarkers was considered when cardiac troponin I or cardiac troponin T ratios were ≥ 10 or when the creatine kinase-isoenzyme MB ratio was ≥ 5.
Statistical AnalysisAll statistical analyses were performed with STATA RELEASE 12 software (StataCorp LP, College Station, Texas, United States). Continuous variables are presented as mean ± standard deviation or median [interquartile range]; categorical variables are presented as numbers and percentages. Sensitivity, specificity, positive predictive value, negative predictive value and efficiency for STEMI equivalent were calculated. Efficiency is a parameter that expresses the percentage of correct classifications by a diagnostic test. The 95% confidence intervals were obtained with the Wald method or, when appropriate, with the Wilson method. Differences in sensitivity and specificity between algorithms were tested with the McNemar test.14 Comparisons between groups were undertaken using the chi-square test or the Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables that were not normally distributed. Differences were considered statistically significant at the P < .05 level.
ResultsBaseline Characteristics and Data From Hospital AdmissionBetween October 2009 and December 2014, 10 122 patients were referred for pPCI. In 251 patients (2.5%), the protocol for pPCI was activated for LBBB. Of these, 106 patients were excluded from the present study due to lack of an available copy of the initial ECG (75 patients) or absence of true complete LBBB in the initial ECG (31 patients). Therefore, the study population consisted of 145 patients.
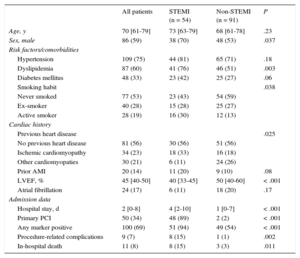
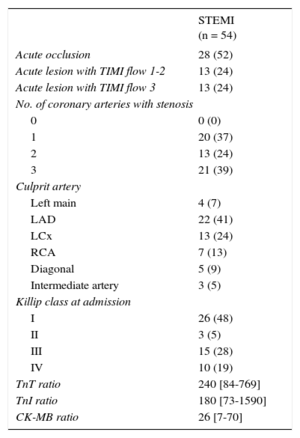
Baseline characteristics are summarized in Table 2. Among the 145 patients, 54 (37%) had STEMI. Among STEMI patients (Table 3), 41 (76%) had a complete acute occlusion or reduced TIMI flow of the culprit coronary artery. In 4 patients (7%), the culprit lesion was found in the left main artery. A high proportion of patients with STEMI presented with Killip class III or IV (47%). Peak levels of biomarkers were clearly elevated in patients with STEMI: the median troponin I ratio was 240 [interquartile range, 84-769], the median TnT ratio was 180 [interquartile range, 73-1590], and the median creatine kinase-isoenzyme MB ratio was 26 [interquartile range, 7-70].
Baseline Characteristics of the Study Population
| All patients | STEMI (n = 54) | Non-STEMI (n = 91) | P | |
|---|---|---|---|---|
| Age, y | 70 [61-79] | 73 [63-79] | 68 [61-78] | .23 |
| Sex, male | 86 (59) | 38 (70) | 48 (53) | .037 |
| Risk factors/comorbidities | ||||
| Hypertension | 109 (75) | 44 (81) | 65 (71) | .18 |
| Dyslipidemia | 87 (60) | 41 (76) | 46 (51) | .003 |
| Diabetes mellitus | 48 (33) | 23 (42) | 25 (27) | .06 |
| Smoking habit | .038 | |||
| Never smoked | 77 (53) | 23 (43) | 54 (59) | |
| Ex-smoker | 40 (28) | 15 (28) | 25 (27) | |
| Active smoker | 28 (19) | 16 (30) | 12 (13) | |
| Cardiac history | ||||
| Previous heart disease | .025 | |||
| No previous heart disease | 81 (56) | 30 (56) | 51 (56) | |
| Ischemic cardiomyopathy | 34 (23) | 18 (33) | 16 (18) | |
| Other cardiomyopaties | 30 (21) | 6 (11) | 24 (26) | |
| Prior AMI | 20 (14) | 11 (20) | 9 (10) | .08 |
| LVEF, % | 45 [40-50] | 40 [33-45] | 50 [40-60] | < .001 |
| Atrial fibrillation | 24 (17) | 6 (11) | 18 (20) | .17 |
| Admission data | ||||
| Hospital stay, d | 2 [0-8] | 4 [2-10] | 1 [0-7] | < .001 |
| Primary PCI | 50 (34) | 48 (89) | 2 (2) | < .001 |
| Any marker positive | 100 (69) | 51 (94) | 49 (54) | < .001 |
| Procedure-related complications | 9 (7) | 8 (15) | 1 (1) | .002 |
| In-hospital death | 11 (8) | 8 (15) | 3 (3) | .011 |
AMI, acute myocardial infarction; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
The chi-square or Fisher exact test, when appropriate, were used to calculate differences between proportions; the Mann-Whitney U test was used to calculate differences between medians.
Data are expressed as No. (%) or median [interquartile range].
Angiographic and Clinical Characteristics of ST-segment Elevation Myocardial Infarction Patients
| STEMI (n = 54) | |
|---|---|
| Acute occlusion | 28 (52) |
| Acute lesion with TIMI flow 1-2 | 13 (24) |
| Acute lesion with TIMI flow 3 | 13 (24) |
| No. of coronary arteries with stenosis | |
| 0 | 0 (0) |
| 1 | 20 (37) |
| 2 | 13 (24) |
| 3 | 21 (39) |
| Culprit artery | |
| Left main | 4 (7) |
| LAD | 22 (41) |
| LCx | 13 (24) |
| RCA | 7 (13) |
| Diagonal | 5 (9) |
| Intermediate artery | 3 (5) |
| Killip class at admission | |
| I | 26 (48) |
| II | 3 (5) |
| III | 15 (28) |
| IV | 10 (19) |
| TnT ratio | 240 [84-769] |
| TnI ratio | 180 [73-1590] |
| CK-MB ratio | 26 [7-70] |
CK-MB, creatine kinase-isoenzyme MB; LAD, left anterior descendent artery; LCx, left circumflex artery; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis In Myocardial Infarction; TnI, troponin I; TnT, troponin T.
Data are expressed as No. (%) or median [interquartile range].
Primary PCI was performed in 48 (89%) patients with STEMI. Among the remaining 6 patients, 2 patients of advanced age with severe comorbidities and a culprit lesion with TIMI 3 flow were managed conservatively and the culprit lesion was not considered suitable for percutaneous revascularization in 4 patients (1 occlusion in the distal segment of the LAD, 1 lesion with TIMI 3 flow in the distal LAD, and 2 lesions with TIMI flow 1/2 in a diagonal branch). Procedure-related complications occurred in 9 patients (7%): 1 fatal bleeding, 1 coronary dissection, 2 failed PCI, and 5 episodes of acute renal failure (1 in a patient without STEMI).
Patients with STEMI had significantly higher in-hospital mortality (15% vs 4%; P = .011). Except for a patient who died from bleeding after pPCI, all other deaths in the STEMI group were cardiac deaths, mainly due to cardiogenic shock. By contrast, 2 out of 3 deaths among patients without STEMI were due to postanoxic encephalopathy; the third death was due to cardiogenic shock in a patient who had been admitted after a resuscitated cardiac arrest.
Three patients from the non-STEMI group had an acute coronary syndrome (ACS) (acute lesion and positive biomarkers) but did not meet the criteria for a STEMI equivalent. All 3 patients underwent revascularization (2 with pPCI and 1 with urgent cardiac surgery).
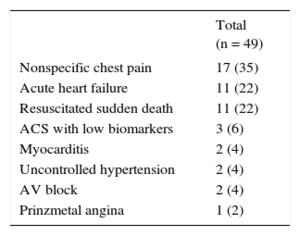
Among patients without STEMI, 49 (54%) had at least 1 cardiac biomarker measurement above the upper limit; their discharge diagnoses are listed in Table 4. Among these patients, 17 (35%) had low biomarker levels without a typical curve and with no evidence of cardiac or extracardiac disease, and were therefore discharged as nonspecific chest pain.
Diagnoses of Non—ST-segment Elevation Myocardial Infarction Patients With Positive Cardiac Biomarkers
| Total (n = 49) | |
|---|---|
| Nonspecific chest pain | 17 (35) |
| Acute heart failure | 11 (22) |
| Resuscitated sudden death | 11 (22) |
| ACS with low biomarkers | 3 (6) |
| Myocarditis | 2 (4) |
| Uncontrolled hypertension | 2 (4) |
| AV block | 2 (4) |
| Prinzmetal angina | 1 (2) |
ACS, acute coronary syndrome; AV, atrioventricular.
Data are expressed as No. (%).
There was complete agreement between the 2 cardiologists that analyzed the ECGs with respect to Sgarbossa criteria and 1 case of disagreement (0.7%) with the Smith I and II rules. The diagnostic performance of each criterion and algorithm are presented in Table 5. Among all Sgarbossa criteria considered alone and the 2 Sgarbossa algorithms, the rule of Sgarbossa score ≥ 3 showed the highest efficiency (74%). A Sgarbossa score ≥ 3 had a sensitivity of 35% and a specificity of 98% for the diagnosis of a STEMI equivalent. Among patients with a culprit lesion located in the left main coronary artery, just 1 of 4 (25%) had a Sgarbossa score ≥ 3. With respect to score ≥ 3, a Sgarbossa score ≥ 2 had significantly higher sensitivity (48%, P = .02) but lower specificity (81%, P < .001).
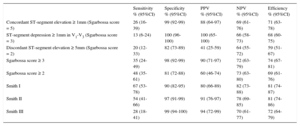
Diagnostic Performance of Sgarbossa Criteria and Sgarbossa and Smith Algorithms for ST-segment Elevation Myocardial Infarction Equivalent
| Sensitivity % (95%CI) | Specificity % (95%CI) | PPV % (95%CI) | NPV % (95%CI) | Efficiency % (95%CI) | |
|---|---|---|---|---|---|
| Concordant ST-segment elevation ≥ 1mm (Sgarbossa score = 5) | 26 (16-39) | 99 (92-99) | 88 (64-97) | 69 (61-76) | 71 (63-78) |
| ST-segment depression ≥ 1mm in V1-V3 (Sgarbossa score = 3) | 13 (6-24) | 100 (96-100) | 100 (65-100) | 66 (58-73) | 68 (60-75) |
| Discordant ST-segment elevation ≥ 5mm (Sgarbossa score = 2) | 20 (12-33) | 82 (73-89) | 41 (25-59) | 64 (55-72) | 59 (51-67) |
| Sgarbossa score ≥ 3 | 35 (24-49) | 98 (92-99) | 90 (71-97) | 72 (63-79) | 74 (67-81) |
| Sgarbossa score ≥ 2 | 48 (35-61) | 81 (72-88) | 60 (46-74) | 73 (63-80) | 69 (61-76) |
| Smith I | 67 (53-78) | 90 (82-95) | 80 (66-89) | 82 (73-88) | 81 (74-87) |
| Smith II | 54 (41-66) | 97 (91-99) | 91 (76-97) | 78 (69-85) | 81 (74-86) |
| Smith III | 28 (18-41) | 99 (94-100) | 94 (72-99) | 70 (61-77) | 72 (64-79) |
95%CI, 95% confidence interval; NPV, negative predictive value; PPV, positive predictive value.
The Smith I rule showed the highest sensitivity (67%), significantly higher than both a Sgarbossa score ≥ 3 (P < .001) and a Sgarbossa score ≥ 2 (P = .01), but still suboptimal; its specificity was 90%, significantly lower than a Sgarbossa score ≥ 3 (P = .02) and not statistically different from a Sgarbossa score ≥ 2.
The Smith II rule, although showing good specificity (97%), had lower sensitivity (54%), comparable to that of a Sgarbossa score ≥ 2 (P = .6). The Smith III rule was limited by the lowest sensitivity (27%).
The overall efficiency of the rules ranged from 69% for a Sgarbossa score ≥ 2 to 81% for the Smith I rule.
If the Smith I or Smith II rules had been used to guide therapeutic management, 18 (33%) or 25 (46%) patients with STEMI, respectively, would not have received a pPCI.
Moreover, patients with STEMI and negative Smith rules often had a very severe AMI, with clinical presentation and a mortality rate similar to those of patients with STEMI and positive Smith I rule (Table 6). Out of 4 patients with the culprit lesion in the left main artery, 2 (50%) had negative Smith I rule and 3 (75%) had a negative Smith II rule.
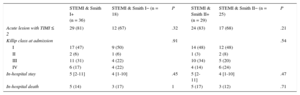
Characteristics of ST-segment Elevation Myocardial Infarction Patients, According to the Positivity of Smith Rules
| STEMI & Smith I+ (n = 36) | STEMI & Smith I− (n = 18) | P | STEMI & Smith II+ (n = 29) | STEMI & Smith II− (n = 25) | P | |
|---|---|---|---|---|---|---|
| Acute lesion with TIMI ≤ 2 | 29 (81) | 12 (67) | .32 | 24 (83) | 17 (68) | .21 |
| Killip class at admission | .91 | .54 | ||||
| I | 17 (47) | 9 (50) | 14 (48) | 12 (48) | ||
| II | 2 (6) | 1 (6) | 1 (3) | 2 (8) | ||
| III | 11 (31) | 4 (22) | 10 (34) | 5 (20) | ||
| IV | 6 (17) | 4 (22) | 4 (14) | 6 (24) | ||
| In-hospital stay | 5 [2-11] | 4 [1-10] | .45 | 5 [2-11] | 4 [1-10] | .47 |
| In-hospital death | 5 (14) | 3 (17) | 1 | 5 (17) | 3 (12) | .71 |
STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis In Myocardial Infarction.
Chi-square or the Fisher exact test, when appropriate, were used to calculate differences between proportions; the Mann-Whitney U test was used to calculate differences between medians.
Data are expressed as No. (%) or median [interquartile range].
None of the 3 patients with AMI and low peak level of cardiac biomarkers had a positive Smith I or II rule; therefore, inclusion of these patients in the analysis would reduce the sensitivity of Smith rules I and II to 63% and 51%, respectively. The results of all criteria and algorithms when applied to the whole group of patients with an ACS, including those not fulfilling STEMI criteria, are presented in Table 7.
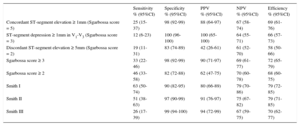
Diagnostic Performance of Sgarbossa Criteria and Sgarbossa and Smith Algorithms for all Acute Coronary Syndrome Patients
| Sensitivity % (95%CI) | Specificity % (95%CI) | PPV % (95%CI) | NPV % (95%CI) | Efficiency % (95%CI) | |
|---|---|---|---|---|---|
| Concordant ST-segment elevation ≥ 1mm (Sgarbossa score = 5) | 25 (15-37) | 98 (92-99) | 88 (64-97) | 67 (58-74) | 69 (61-76) |
| ST-segment depression ≥ 1mm in V1-V3 (Sgarbossa score = 3) | 12 (6-23) | 100 (96-100) | 100 (65-100) | 64 (55-71) | 66 (57-73) |
| Discordant ST-segment elevation ≥ 5mm (Sgarbossa score = 2) | 19 (11-31) | 83 (74-89) | 42 (26-61) | 61 (52-70) | 58 (50-66) |
| Sgarbossa score ≥ 3 | 33 (22-46) | 98 (92-99) | 90 (71-97) | 69 (61-77) | 72 (65-79) |
| Sgarbossa score ≥ 2 | 46 (33-58) | 82 (72-88) | 62 (47-75) | 70 (60-78) | 68 (60-75) |
| Smith I | 63 (50-74) | 90 (82-95) | 80 (66-89) | 79 (70-86) | 79 (72-85) |
| Smith II | 51 (38-63) | 97 (90-99) | 91 (76-97) | 75 (67-82) | 79 (71-85) |
| Smith III | 26 (17-39) | 99 (94-100) | 94 (72-99) | 67 (59-75) | 70 (62-77) |
95%CI, 95% confidence interval; NPV, negative predictive value; PPV, positive predictive value.
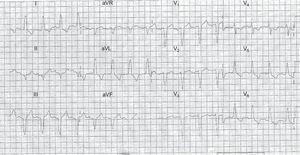
An example of a STEMI patient with negative Smith rules is shown in the Figure.
DiscussionTo our knowledge, this is the first study that sought to assess the ECG algorithms proposed by Smith et al.12 in a cohort of patients with LBBB referred for pPCI.
The main result of the present study is that, although Smith rule I significantly improves the diagnostic efficacy of the Sgarbossa rules, it is still associated with suboptimal sensitivity; at present, it cannot be recommended as the standard tool to diagnose STEMI and indicate pPCI.
Diagnosis of Myocardial Infarction in the Presence of Left Bundle Branch Block: An Enduring PuzzleThe ST-segment deviation in patients with LBBB has some typical characteristics: the deviation is usually opposite to the main axis of the QRS (ie, discordant) and tends to be proportional to the voltage of the QRS. Acute ischemia is associated with an increase in the discordant deviation.
These repolarization abnormalities hinder the diagnosis of AMI and, although several electrocardiographic criteria have been proposed,1–3 none have proved sufficiently reliable to be recommended in clinical practice. Although Sgarbossa criteria represent an important step forward, they are limited by low sensitivity4,15–21: a meta-analysis found an overall sensitivity of 20% for a Sgarbossa score ≥ 3.5
A limitation of both the original Sgarbossa report and subsequent validation studies was that they used only cardiac biomarker levels and not coronary angiography to establish the diagnosis of AMI.
The study by Smith et al.12 was the first to evaluate the Sgarbossa algorithms in the setting of pPCI; these authors found a sensitivity of 52% for a Sgarbossa score ≥ 3 for the diagnosis of STEMI equivalent. In line with previous studies, the present report found that a Sgarbossa score ≥ 3 showed excellent specificity (96%) but low sensitivity (33%).
A limitation of Sgarbossa rules might be that they do not take into account the proportionality between discordant ST-segment deviation and QRS amplitude. The new criteria proposed by Smith are based on ST/QRS proportionality and, in the original report, they achieved an excellent diagnostic performance.12
Unfortunately, our results do not confirm the expectations generated by the original article. Smith rules did increase the diagnostic efficacy of Sgarbossa criteria and, indeed, the Smith I rule had the highest efficiency (81%) among the algorithms analyzed. Smith algorithms also showed good specificity. However, they still displayed suboptimal sensitivity, far below that of the original report (67% vs 91% for the Smith I rule).
A recent validation study of the Smith I rule22 observed a sensitivity of 80%, which was lower than the value found in the derivation sample but higher than that in the present report. However, some important differences exist between the aforementioned study and ours. The study by Meyers et al.22 was a case-control study that also included patients without suspected AMI and who had not undergone emergent coronary angiography; in contrast, the present report is a cohort study of patients referred for emergent catheterization, which is probably the ideal setting to test diagnostic criteria for STEMI equivalent. Moreover, in the study by Meyers et al.,22 2 patients were included in the STEMI group, although they had not undergone coronary angiography and, in the present work, we have shown that a diagnosis based only upon cardiac biomarkers may be misleading. Finally, that report was not a completely external validation, since the authors included patients from the Minneapolis Heart Institute, as in the original report.
According to our results, Smith algorithms may be a useful tool but are not the definitive solution to the complex problem of the ECG diagnosis of AMI in patients with LBBB. Moreover, by employing the ratio between ST-segment elevation and QRS amplitude with cutoff values of −0.25 and −0.30, these criteria may become too complex to be calculated, especially in the emergent setting of suspected AMI.
Finally, among patients with STEMI equivalent, the positivity of Smith rules did not identify higher-risk patients; in fact, the severity of the AMI was similar between patients with positive and negative Smith algorithms. Of note, Smith rules were not originally proposed to identify high-risk patients.
However, the strategy of adding a rule based on the concept of proportionality between ST-segment deviation and QRS amplitude improves the diagnosis of AMI in the presence of LBBB and should be further explored.
Emergent Revascularization in Patients With Left Bundle Branch Block and Suspected Acute Myocardial InfarctionBased on a meta-analysis showing that fibrinolysis is associated with increased survival in patients with LBBB or right bundle branch block and suspected AMI,23 guidelines have traditionally recommended emergent reperfusion for patients with chest pain and new or presumably new LBBB.6,7
Remarkably, no data are available about the effect of pPCI in patients with LBBB, since these patients have been systematically excluded from pPCI trials.
Increasing evidence suggests that LBBB is a major cause of false activation of the catheterization laboratory for pPCI. Among patients with chest pain and LBBB referred for pPCI, only between 22% and 26% actually benefit from emergent revascularization.8–10 In view of such results, the 2013 guidelines of the American Heart Association11 stated that LBBB should not be considered diagnostic of AMI in isolation.
In line with previous reports, in the present series, almost two thirds of the patients underwent an unnecessary emergent coronary angiogram.
The absence of a reliable ECG diagnosis of AMI in the presence of LBBB is a pressing clinical problem since any strategy chosen has significant limitations. To treat all of these cases as STEMI implies that a majority of patients with no ACS will be unnecessarily exposed to a costly protocol and to the risks of either fibrinolysis or aggressive anticoagulation and antiplatelet therapy in association with coronary angiography.
On the other hand, considering LBBB as nondiagnostic may delay the time to reperfusion in those patients who are really experiencing an AMI. This could be especially detrimental since AMIs associated with new LBBB are usually severe.24,25 Data from our population with STEMI and new or presumably new LBBB confirm that this is a very high-risk subgroup.
In the absence of reliable electrocardiographic criteria, clinical judgement, cardiac biomarkers and echocardiography may be of help. However, clinical presentation can be atypical and echocardiography is not always available on-site at the secondary hospitals or ambulances that are often responsible for deciding whether a patient needs emergent reperfusion or not.
Cardiac biomarkers also have some important drawbacks. First, there is a time delay between symptom onset and biomarker release. Second, elevation of cardiac biomarkers can be secondary to causes other than ACS; moreover, even when elevation occurs in the context of ACS, it does not always mean that emergent reperfusion is required. In the present study, among patients without STEMI, 54% had abnormal levels of at least 1 cardiac biomarker.
In summary, prompt diagnosis of AMI in the presence of LBBB remains challenging. In this scenario, the search for new ECG algorithms should be encouraged to avoid unnecessary application of costly protocols and aggressive treatments in the vast majority of patients. At the same time, new ECG algorithms should enable timely emergent reperfusion in a subgroup of high-risk patients.
LimitationsThe main limitation of the present study is its observational nature. Because diagnosis of STEMI is based on electrocardiographic criteria7 that are not applicable in patients with LBBB, a clinical equivalent of STEMI had to be defined for the purposes of the present study; the definition of a STEMI equivalent has inherent limitations. Like Smith et al.,12 we tried to overcome these limitations by using angiographic and biochemical parameters. The choice of these parameters was based both on the Smith report and on data from large studies that reported angiographic and biochemical data about STEMI and non-STEMI patients.26–30 Complete acute occlusion of a coronary artery should be considered as STEMI equivalent. However, especially after pretreatment with unfractionated heparin, between one fourth and one third of STEMI patients have a patent culprit artery at the time of pPCI.26,31 In cases of patent culprit artery, cardiac biomarkers that are clearly associated with infarct size26 may be helpful in detecting ACS with relevant myocardial injury that are likely to benefit from pPCI. In general, STEMI is associated with higher biomarker release than non-STEMI, but considerable overlap exists.27 Considering a biomarker ratio as the peak level divided by the upper normal limit, 25% of STEMI patients were found to have a cardiac troponin I ratio lower than 4527 and 11% fitted into a category of low cardiac troponin defined by a lower limit of cardiac troponin ratio of 10.28 The creatine kinase-isoenzyme MB ratio is usually lower than the cardiac troponin I27,29,30 ratio and the upper limit of the first quartile for the creatine kinase-isoenzyme MB ratio in STEMI was found to be 8 in a previous study that included the largest population investigated so far.27
Smith et al.12 considered that a diagnosis of STEMI could be reasonably assumed either in the presence of an acutely occluded coronary artery (TIMI flow 0-1) or when an acute nonocclusive coronary lesion was present in association with a significant increase in cardiac troponin I levels. They arbitrarily established a cutoff of 10 ng/mL for peak 24-hour cardiac troponin I levels as relevant for the diagnosis of STEMI. Of note, the different hospitals participating in the study had varying upper normal limits for cardiac troponin I (between 0.1 ng/mL and 0.6 ng/mL).
Because each hospital in the present study had different reference values for cardiac biomarkers, we considered that using a ratio between the biomarker level and the reference value was a more standardized and reproducible method. Moreover, in their validation study, Meyers et al.22 also included patients with a cardiac troponin T ratio > 10. The selection criteria used in the present study yielded a population of STEMI-equivalent patients whose angiographic and biochemical characteristics were similar to those of published STEMI series27,28,31 and presented with high-risk AMI. Using biomarker ratios with the aforementioned cutoff, only 3 patients in our STEMI population had an absolute value of troponin T or TnI inferior to the lower limit considered in the validation study by Meyers et al.22; 2 of these patients had positive Smith rules and therefore this issue cannot be considered responsible for the reported difference in the sensitivity of Smith rules. There are several differences in the study design between the present report and the study by Smith et al.12 The former is an observational cohort study and the latter is a case-control study. The control population in Smith's report was not necessarily referred for pPCI and had not always undergone a coronary angiogram. By contrast, all of our patients were referred for pPCI and had a coronary angiogram. Smith et al.12 excluded patients with respiratory failure, heart rate greater than 130 bpm, hyperkalemia and severe hypertension while our report is an “all comers” study. A population of unselected patients with LBBB referred for pPCI is probably the most realistic setting in which to identify ECG algorithms for the diagnosis of STEMI and indeed it is the population that would benefit most from an optimization of the diagnostic process.
Percutaneous coronary intervention can produce a further increase in cardiac biomarker release that might have led to a misclassification of some patients with a non—ST-segment elevation ACS in the STEMI group. However, given the low number of procedural complications concerning the coronary arteries and the very high levels of cardiac biomarkers observed in STEMI patients, it seems unlikely that this limitation significantly affected the results of the study; moreover, this potential confounder was not taken into account in the Smith report.
A final limitation of the study is the relatively small sample size, due to the low prevalence of LBBB among patients referred for pPCI and to the lack of an initial ECG in some of them. However, this limitation is also applicable to previous studies on the same issue.
ConclusionsAn ECG diagnosis of STEMI in the presence of LBBB remains a challenge. Sgarbossa criteria and algorithms are associated with high specificity but low sensitivity. Smith rules represent an important step forward; however, they are still limited by suboptimal sensitivity. Further research to improve the diagnostic efficacy of ECG in this setting should be encouraged to avoid unnecessary and aggressive treatments for a majority of patients and, at the same time, provide timely reperfusion to the high-risk subgroup of STEMI patients with LBBB.
CONFLICTS OF INTERESTNone declared.
- –
Diagnosis of myocardial infarctions is complex in the presence of LBBB.
- –
Sgarbossa criteria are limited by low sensitivity.
- –
Smith criteria seem promising.
- –
Smith criteria perform better than Sgarbossa rules.
- –
However, Smith criteria have suboptimal sensitivity.
- –
Smith criteria do not identify higher-risk patients. Patients with AMI and negative Smith criteria often have very severe AMI.