The equations used in the general population to calculate cardiovascular risk are not useful in genetic hypercholesterolemia (GH). Carotid plaque detection has proved useful in cardiovascular prediction and risk reclassification but there have been no studies of its usefulness in GH. The aim of this study was to determine the association between the presence of carotid artery plaque and the occurrence of cardiovascular events in patients with GH.
MethodsThis study included 1778 persons with GH. The mean follow-up until the occurrence of cardiovascular events was 6.26 years. At presentation, the presence of carotid artery plaque was studied by high-resolution ultrasound.
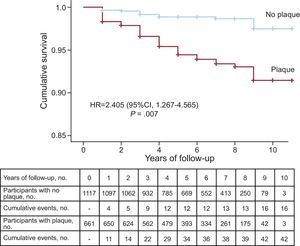
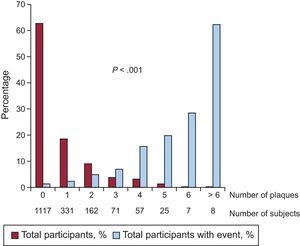
ResultsCarotid artery plaque was found in 661 (37.2%) patients: 31.9% with familial hypercholesterolemia, 39.8% with familial combined hyperlipidemia, 45.5% with dysbetalipoproteinemia, and 43.2% with polygenic hypercholesterolemia. During follow-up, 58 patients had a cardiovascular event. Event rates were 6354/100 000 (95%CI, 4432.4-8275.6) in the group with plaque and 1432/100 000 (95%CI, 730.6-2134.3) in the group without plaque, with significant differences between the 2 groups (P < .001). The relative risk of an event was 4.34 (95CI%, 2.44-7.71; P < .001) times higher in patients with plaque and was 2.40 (95%CI, 1.27-4.56; P = .007) times higher after adjustment for major risk factors. The number of carotid artery plaques was positively associated with the risk of cardiovascular events.
ConclusionsMost cardiovascular events occur in a subgroup of patients who can be identified by carotid plaque detection. These results support the use of plaque screening in this population and should help in risk stratification and treatment in GH.
Keywords
Cardiovascular disease (CVD) is the main cause of mortality and disability in most countries.1 In nearly one third of patients, the first manifestation of CVD is fatal or leaves irreversible sequelae,2,3 and event recurrence remains very high.4,5 Treatment of CVD must be prioritized in primary prevention by intervention stratification according to individual risk.6 In the general population, risk is predicted using equations based on the presence of classic risk factors.7 Although these tools have many limitations,8 they are recommended by scientific societies to determine the risk of CVD.6,9
An exception to the use of primary prevention risk equations are patients with genetic hypercholesterolemia (GH). These equations underestimate the risk of these patients and should not be used in this population, as they are considered to be at high risk of CVD due to very high atherogenic lipoprotein concentrations from birth.6,7,9,10 However, not all types of GH entail the same cardiovascular risk or require the same intervention and, consequently, novel procedures are essential for CVD prediction and risk stratification in these patients.10
Screening for subclinical atherosclerosis has been proposed as a tool to improve the prediction of CVD.11 The marker that has traditionally been used is carotid intima-media thickness (IMT).12 Nevertheless, later studies have shown that it provides little improvement to predictions based exclusively on classic risk factors13 and, therefore, is currently not recommended for use.14 Conversely, the presence of carotid artery plaque is closely associated with the risk of CVD,12 improves risk factor-based prediction,15 and helps reclassify up to 22.7% of participants at intermediate risk.16 The presence of plaque in the carotid artery as a tool to predict CVD has not been previously studied in patients with GH.
To identify the association between the presence of carotid plaques and the appearance of CVD in GH, we prospectively studied a cohort of patients with GH seen in the lipid unit of the Hospital Universitario Miguel Servet in Zaragoza, Spain.
METHODSPatientsA prospective cohort study was conducted, including all patients aged 18 to 80 years and diagnosed with GH who were attended in the unit between January 2006 and December 2014. Patients were diagnosed with GH when total cholesterol or low-density lipoprotein cholesterol (LDL-C) was above the 95th percentile for the Spanish population adjusted by age and sex in the presence or absence of triglycerides > 300mg/dL.17 To diagnose polygenic hypercholesterolemia, the LDL-C concentration had to be above the 90th percentile. In all patients, secondary causes were excluded: body mass index > 35, TSH > 6 mIU/L, creatinine > 2.0 mg/dL, poorly controlled diabetes (glycohemoglobin > 7.5%), cholestasis (direct bilirubin > 1 mg/dL), or use of drugs that favor lipid metabolism disorders. Familial hypercholesterolemia (FH) was diagnosed in patients with LDL-C > 95th percentile with vertical familial transmission of hypercholesterolemia, LDL-C > 95th percentile in at least 1 first-degree relative, and triglycerides < 200mg/dL. Familial combined hyperlipidemia (FCH) was diagnosed in patients with GH and triglycerides > 200 mg/dL, apolipoprotein B > 120 mg/dL, vertical transmission of hyperlipidemia, and at least 1 first-degree relative with total cholesterol or triglyceride levels > 90th percentile.18 In GH, dysbetalipoproteinemia was considered to be homozygous for allele ¿2 or heterozygous for allele p.Arg154Ser of APOE.19 All other patients with GH were diagnosed with polygenic hypercholesterolemia. All participants gave written consent before participating in the protocol, which was approved by the Clinical Research Ethics Committee of Aragón, Spain.
Carotid UltrasoundFollowing inclusion in the study, patients underwent an ultrasound examination of the carotid artery with an Acuson Sequoia (Siemens) ultrasound machine using a 7-MHz probe, and an imaging study was performed on the common carotid artery, bifurcation, or internal bulb and carotid (3 for the right side and 3 for the left). Each image was read to obtain the mean IMT for each territory and to calculate the mean IMT. The same person (A.M. Bea) performed all measurements with the Exetrack software, all of them at end-diastole, simultaneously using an ECG tracing. Plaque was defined as a focal structure protruding in the arterial lumen more than 0.5 mm or more than 50% of IMT from a contiguous area or any IMT ≥ 1.5 mm.20 The total number of plaques was calculated for each participant in the 6 territories examined. Hypoechoic shadows were considered to be calcified plaques.
Genetic StudyIn all patients with a clinical diagnosis of FH or a diagnosis of FCH and total cholesterol levels > 335 mg/dL or apolipoprotein B > 185 mg/dL, the Lipochip platform was used to analyze the LDLR and APOB genes.21 In patients with a clinical diagnosis of FCH who had a pathogenic mutation in LDLR, APOB, or PCSK9, the diagnosis was FH.21APOE exon 4 was sequenced in all participants.19
Definition of Cardiovascular EventThe study endpoint was the compound event of coronary heart disease (coronary death, acute coronary syndrome requiring hospitalization, and coronary revascularization due to angina), stroke (fatal and nonfatal stroke, transient ischemic attack, and carotid revascularization), and peripheral artery disease (lower extremity arterial revascularization).22
Follow-upOnce included, all patients underwent checkups 1 or 2 times a year. Throughout the study, dyslipidemia was treated in accordance with the recommendations of the International Panel on Management of Familial Hypercholesterolemia,23 which establishes the therapeutic target as a reduction in LDL-C and/or non–high-density lipoprotein cholesterol (non–HDL-C), according to risk factors. Since November 2013, the therapeutic target for patients with CVD or diabetes has been amended to LDL-C < 70 mg/dL.10
From 1 September 2015 to 31 May 2016, all participants received checkups through a face-to-face or telephone interview and/or an electronic medical record review. All events were confirmed by the hospital report and/or the death certificate.
Statistical AnalysisData are expressed as mean ± standard deviation for numeric variables with a normal distribution and were analyzed by the Student t test. Variables with a nonnormal distribution are expressed as median [interquartile range] and were analyzed by the Mann-Whitney U test. Qualitative variables are expressed as percentage and were analyzed by the chi-square test. ANOVA and Kruskal-Wallis tests were used to compare nondichotomous categorical variables. Adverse event rates until the completion of follow-up were calculated using Kaplan-Meier estimates, and the groups were compared by log-rank tests. The association between carotid plaque and cardiovascular events was calculated using proportional risks Cox regression. A multivariable Cox regression model was generated and included the following co-variables: age, sex, diabetes, hypertension, smoking, history of CVD, body mass index, LDL-C, HDL-C, C-reactive protein, and type of GH. As an exploratory analysis, these associations were examined in the various GH groups and in participants receiving primary or secondary prevention at baseline. To avoid biases associated with the type of GH, we also conducted a nested case-control study, in which an event-free control adjusted for age, sex, total cholesterol, and type of GH was selected at random for each participant with a cardiovascular event during follow-up.
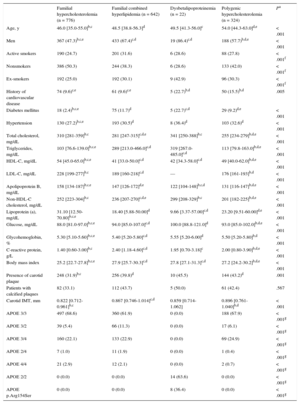
RESULTSTable 1 describes the characteristics of the 1778 patients included in follow-up, listed according to the 4 types of GH. Patients with FH were younger than those with other types of GH. Furthermore, the FH group contained slightly more women (52.7%), although there were more men in the other 3 types of GH, particularly in FCH (67.4%) and dysbetalipoproteinemia (86.4%). Carotid plaques were found in 31.9% of the FH group, 39.8% in FCH, 45.5% in dysbetalipoproteinemia, and 43.2% in polygenic hypercholesterolemia.
Clinical Characteristics of Participants Included in Follow-up According to Type of Genetic Hypercholesterolemia
| Familial hypercholesterolemia (n = 776) | Familial combined hyperlipidemia (n = 642) | Dysbetalipoproteinemia (n = 22) | Polygenic hypercholesterolemia (n = 324) | Pa | |
|---|---|---|---|---|---|
| Age, y | 46.0 [35.0-55.0]b,c | 48.5 [38.8-56.3]d | 49.5 [41.3-56.0]c | 54.0 [44.3-63.0]d,e | < .001 |
| Men | 367 (47.3)b,c,e | 433 (67.4)c,d | 19 (86.4)c,d | 188 (57.7)b,d,e | < .001 |
| Active smokers | 190 (24.7) | 201 (31.6) | 6 (28.6) | 88 (27.8) | < .001f |
| Nonsmokers | 386 (50.3) | 244 (38.3) | 6 (28.6) | 133 (42.0) | < .001f |
| Ex-smokers | 192 (25.0) | 192 (30.1) | 9 (42.9) | 96 (30.3) | < .001f |
| History of cardiovascular disease | 74 (9.6)c,e | 61 (9.6)c,e | 5 (22.7)b,d | 50 (15.5)b,d | .005 |
| Diabetes mellitus | 18 (2.4)b,c,e | 75 (11.7)d | 5 (22.7)c,d | 29 (9.2)d,e | < .001 |
| Hypertension | 130 (27.2)b,c,e | 193 (30.5)d | 8 (36.4)d | 103 (32.6)d | < .001 |
| Total cholesterol, mg/dL | 310 [281-359]b,c | 281 [247-315]c,d,e | 341 [250-388]b,c | 255 [234-279]b,d,e | < .001 |
| Triglycerides, mg/dL | 103 [76.6-139.0]b,c,e | 289 [213.0-466.0]c,d | 319 [267.0-485.0]c,d | 113 [79.8-163.0]b,d,e | < .001 |
| HDL-C, mg/dL | 54 [45.0-65.0]b,c,e | 41 [33.0-50.0]c,d | 42 [34.3-58.0]c,d | 49 [40.0-62.0]b,d,e | < .001 |
| LDL-C, mg/dL | 228 [199-277]b,c | 189 [160-218]c,d | — | 176 [161-193]b,d | < .001 |
| Apolipoprotein B, mg/dL | 158 [134-187]b,c,e | 147 [126-172]d,e | 122 [104-148]b,c,d | 131 [116-147]b,d,e | < .001 |
| Non-HDL-C cholesterol, mg/dL | 252 [223-304]b,c | 236 [207-270]c,d,e | 299 [208-329]b,c | 201 [182-225]b,d,e | < .001 |
| Lipoprotein (a), mg/dL | 31.10 [12.50-70.80]b,c,e | 18.40 [5.88-50.00]d | 9.66 [3.37-57.00]c,d | 23.20 [9.51-60.00]d,e | < .001 |
| Glucose, mg/dL | 88.0 [81.0-97.0]b,c,e | 94.0 [85.0-107.0]c,d | 100.0 [88.8-121.0]d | 93.0 [85.0-102.0]b,d,e | < .001 |
| Glycohemoglobin, % | 5.30 [5.10-5.60]b,c,e | 5.40 [5.20-5.80]c,d | 5.55 [5.20-6.00]d | 5.50 [5.20-5.80]b,d | < .001 |
| C-reactive protein, g/L | 1.40 [0.60-3.00]b,c | 2.40 [1.18-4.60]c,d | 1.95 [0.70-3.18]c | 2.00 [0.80-3.90]b,d,e | < .001 |
| Body mass index | 25.2 [22.7-27.8]b,c,e | 27.9 [25.7-30.3]c,d | 27.8 [27.1-31.3]c,d | 27.2 [24.2-30.2]b,d,e | < .001 |
| Presence of carotid plaque | 248 (31.9)b,c | 256 (39.8)d | 10 (45.5) | 144 (43.2)d | .001 |
| Patients with calcified plaques | 82 (33.1) | 112 (43.7) | 5 (50.0) | 61 (42.4) | .567 |
| Carotid IMT, mm | 0.822 [0.712-0.961]b,c | 0.867 [0.746-1.014]c,d | 0.859 [0.714-1.062] | 0.896 [0.761-1.040]b,d | < .001 |
| APOE 3/3 | 497 (68.6) | 360 (61.9) | 0 (0.0) | 188 (67.9) | < .001g |
| APOE 3/2 | 39 (5.4) | 66 (11.3) | 0 (0.0) | 17 (6.1) | < .001g |
| APOE 3/4 | 160 (22.1) | 133 (22.9) | 0 (0.0) | 69 (24.9) | < .001g |
| APOE 2/4 | 7 (1.0) | 11 (1.9) | 0 (0.0) | 1 (0.4) | < .001g |
| APOE 4/4 | 21 (2.9) | 12 (2.1) | 0 (0.0) | 2 (0.7) | < .001g |
| APOE 2/2 | 0 (0.0) | 0 (0.0) | 14 (63.6) | 0 (0.0) | < .001g |
| APOE p.Arg154Ser | 0 (0.0) | 0 (0.0) | 8 (36.4) | 0 (0.0) | < .001g |
APOE, apolipoprotein E; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; LDL-C, low-density lipoprotein cholesterol.
Data are expressed as No. (%) or median [interquartile range].
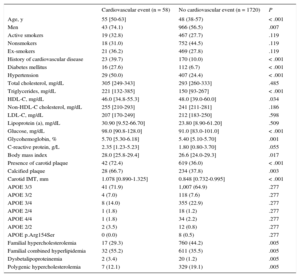
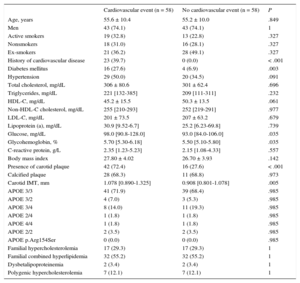
Mean follow-up was 6.26 (range, 1-10) years, for a total of 11 130 patient-years of follow-up. During follow-up, 58 patients had a cardiovascular event: 48 coronary events (4 sudden deaths, 10 nonfatal myocardial infarctions, 26 acute coronary syndromes with or without revascularization, 8 coronary revascularizations); 8 cerebrovascular events (4 ischemic strokes and 4 carotid revascularizations) and 2 patients with arteriopathy in the lower limbs (both revascularizations). No patients were lost to follow-up. Table 2 lists the clinical characteristics of participants with and without events during follow-up. Patients who experienced a cardiovascular event during follow-up had more classic risk factors (age, male sex, diabetes, hypertension, high triglyceride levels, low HDL-C levels, and high body mass index) than patients without an event. Total cholesterol, LDL-C, non–HDL-C, and apolipoprotein B levels did not differ between the 2 groups. A total of 72.4% of participants with cardiovascular events during follow-up had carotid plaques at baseline compared with 36.0% of participants with no events (P < .001), ie, 72.4% of events appeared in 37.2% of patients with plaque. Additionally, among patients with plaque, calcification was detected more often in patients who later had an event than in those who did not (66.7% vs 37.8%; P = .003).
Clinical and Laboratory Characteristics of Patients With and Without a Cardiovascular Event During Follow-up
| Cardiovascular event (n = 58) | No cardiovascular event (n = 1720) | P | |
|---|---|---|---|
| Age, y | 55 [50-63] | 48 (38-57) | < .001 |
| Men | 43 (74.1) | 966 (56.5) | .007 |
| Active smokers | 19 (32.8) | 467 (27.7) | .119 |
| Nonsmokers | 18 (31.0) | 752 (44.5) | .119 |
| Ex-smokers | 21 (36.2) | 469 (27.8) | .119 |
| History of cardiovascular disease | 23 (39.7) | 170 (10.0) | < .001 |
| Diabetes mellitus | 16 (27.6) | 112 (6.7) | < .001 |
| Hypertension | 29 (50.0) | 407 (24.4) | < .001 |
| Total cholesterol, mg/dL | 305 [249-343] | 293 [260-333] | .485 |
| Triglycerides, mg/dL | 221 [132-385] | 150 [93-267] | < .001 |
| HDL-C, mg/dL | 46.0 [34.8-55.3] | 48.0 [39.0-60.0] | .034 |
| Non-HDL-C cholesterol, mg/dL | 255 [210-293] | 241 [211-281] | .186 |
| LDL-C, mg/dL | 207 [170-249] | 212 [183-250] | .598 |
| Lipoprotein (a), mg/dL | 30.90 [9.52-66.70] | 23.80 [8.90-61.20] | .509 |
| Glucose, mg/dL | 98.0 [90.8-128.0] | 91.0 [83.0-101.0] | < .001 |
| Glycohemoglobin, % | 5.70 [5.30-6.18] | 5.40 [5.10-5.70] | .001 |
| C-reactive protein, g/L | 2.35 [1.23-5.23] | 1.80 [0.80-3.70] | .055 |
| Body mass index | 28.0 [25.8-29.4] | 26.6 [24.0-29.3] | .017 |
| Presence of carotid plaque | 42 (72.4) | 619 (36.0) | < .001 |
| Calcified plaque | 28 (66.7) | 234 (37.8) | .003 |
| Carotid IMT, mm | 1.078 [0.890-1.325] | 0.848 [0.732-0.995] | < .001 |
| APOE 3/3 | 41 (71.9) | 1,007 (64.9) | .277 |
| APOE 3/2 | 4 (7.0) | 118 (7.6) | .277 |
| APOE 3/4 | 8 (14.0) | 355 (22.9) | .277 |
| APOE 2/4 | 1 (1.8) | 18 (1.2) | .277 |
| APOE 4/4 | 1 (1.8) | 34 (2.2) | .277 |
| APOE 2/2 | 2 (3.5) | 12 (0.8) | .277 |
| APOE p.Arg154Ser | 0 (0.0) | 8 (0.5) | .277 |
| Familial hypercholesterolemia | 17 (29.3) | 760 (44.2) | .005 |
| Familial combined hyperlipidemia | 32 (55.2) | 611 (35.5) | .005 |
| Dysbetalipoproteinemia | 2 (3.4) | 20 (1.2) | .005 |
| Polygenic hypercholesterolemia | 7 (12.1) | 329 (19.1) | .005 |
APOE, apolipoprotein E; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; LDL-C, low-density lipoprotein cholesterol.
Data are expressed as No. (%) or median [interquartile range].
The event rate was 6354/100 000 (95% confidence interval [95%CI], 4432.4-8275.6) in patients with plaque and 1432/100 000 (95%CI, 730.6-2134.3) in those without plaque, with a significant difference between the 2 groups (P < .001) (Figure 1). The risk of an event was 4.34 times higher (95%CI, 2.44-7.71; P < .001) in patients with plaque. The curves began to show separation already in the first year of follow-up and continued to rise gradually until the end. The total number of plaques in each participant was closely associated with the probability of an event, which progressively rose from 2.4% in patients with only 1 plaque to 62.5% in patients with more than 6 plaques (Figure 2).
The variables prospectively associated with an event were age, sex, and a previous event (Table 3). When the presence of plaques was introduced into the model with these variables, patients with plaques had a risk of an event, adjusted by the above variables, that was 2.405 (95%CI, 1.267-4.565; P = .007) times higher. When only participants with no previous cardiovascular events were selected, the presence of plaques represented a risk that was 2.76 (95%CI, 1.40-5.43; P = .003) times higher of experiencing a new cardiovascular event in primary prevention. This risk was 10.5-fold (P = .023) in patients with a previous event and atheromatous carotid plaque, although the confidence interval was high due to the small number of participants.
Prospective Multivariable Cox Regression Analysis of Predictive Factors for an Event
| HR (95%CI) | |
|---|---|
| History of cardiovascular disease | 2.79 (1.56-5.01) |
| Presence of carotid plaque | 2.40 (1.27-4.56) |
| Age | 1.03 (1.00-1.05) |
| Male sex | 1.86 (0.99-3.47) |
95%CI, 95% confidence interval; HR, hazard ratio.
Variables introduced in the model: age, sex, diabetes, hypertension, smoking, history of cardiovascular disease, body mass index, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, C-reactive protein, type of genetic hypercholesterolemia, and presence of carotid plaque; the statistically significant variables remained.
Table 4 lists the clinical characteristics of the case-control study, with randomization adjusted for age, sex, total cholesterol level, and type of GH. A history of previous CVD, diabetes, and hypertension were more common in the patients. Carotid plaques were found in 72.4% of patients compared with 27.6% of controls (hazard ratio [HR] = 6.89; 95%CI, 3.05-15.56; P < .001). The results were similar when the analysis included only patients with a coronary event during follow-up (HR = 5.25; 95%CI, 2.28-12.06; P < .001). However, there were no differences between the 2 groups in the percentage of participants with calcified plaques.
Clinical and Laboratory Characteristics of Patients With an Cardiovascular Event During Follow-up and Their Controls Adjusted by Age, Sex, Total Cholesterol, and Type of Genetic Hypercholesterolemia
| Cardiovascular event (n = 58) | No cardiovascular event (n = 58) | P | |
|---|---|---|---|
| Age, years | 55.6 ± 10.4 | 55.2 ± 10.0 | .849 |
| Men | 43 (74.1) | 43 (74.1) | 1 |
| Active smokers | 19 (32.8) | 13 (22.8) | .327 |
| Nonsmokers | 18 (31.0) | 16 (28.1) | .327 |
| Ex-smokers | 21 (36.2) | 28 (49.1) | .327 |
| History of cardiovascular disease | 23 (39.7) | 0 (0.0) | < .001 |
| Diabetes mellitus | 16 (27.6) | 4 (6.9) | .003 |
| Hypertension | 29 (50.0) | 20 (34.5) | .091 |
| Total cholesterol, mg/dL | 306 ± 80.6 | 301 ± 62.4 | .696 |
| Triglycerides, mg/dL | 221 [132-385] | 209 [111-311] | .232 |
| HDL-C, mg/dL | 45.2 ± 15.5 | 50.3 ± 13.5 | .061 |
| Non-HDL-C cholesterol, mg/dL | 255 [210-293] | 252 [219-291] | .977 |
| LDL-C, mg/dL | 201 ± 73.5 | 207 ± 63.2 | .679 |
| Lipoprotein (a), mg/dL | 30.9 [9.52-6.7] | 25.2 [6.23-69.8] | .739 |
| Glucose, mg/dL | 98.0 [90.8-128.0] | 93.0 [84.0-106.0] | .035 |
| Glycohemoglobin, % | 5.70 [5.30-6.18] | 5.50 [5.10-5.80] | .035 |
| C-reactive protein, g/L | 2.35 [1.23-5.23] | 2.15 [1.08-4.33] | .557 |
| Body mass index | 27.80 ± 4.02 | 26.70 ± 3.93 | .142 |
| Presence of carotid plaque | 42 (72.4) | 16 (27.6) | < .001 |
| Calcified plaque | 28 (68.3) | 11 (68.8) | .973 |
| Carotid IMT, mm | 1.078 [0.890-1.325] | 0.908 [0.801-1.078] | .005 |
| APOE 3/3 | 41 (71.9) | 39 (68.4) | .985 |
| APOE 3/2 | 4 (7.0) | 3 (5.3) | .985 |
| APOE 3/4 | 8 (14.0) | 11 (19.3) | .985 |
| APOE 2/4 | 1 (1.8) | 1 (1.8) | .985 |
| APOE 4/4 | 1 (1.8) | 1 (1.8) | .985 |
| APOE 2/2 | 2 (3.5) | 2 (3.5) | .985 |
| APOE p.Arg154Ser | 0 (0.0) | 0 (0.0) | .985 |
| Familial hypercholesterolemia | 17 (29.3) | 17 (29.3) | 1 |
| Familial combined hyperlipidemia | 32 (55.2) | 32 (55.2) | 1 |
| Dysbetalipoproteinemia | 2 (3.4) | 2 (3.4) | 1 |
| Polygenic hypercholesterolemia | 7 (12.1) | 7 (12.1) | 1 |
APOE, apolipoprotein E; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; LDL-C, low-density lipoprotein cholesterol.
Data are expressed as No. (%), mean ± standard deviation, or median [interquartile range].
This is the first study to prospectively analyze the association between the presence of arteriosclerosis plaque detected by carotid ultrasound in participants with GH and the appearance of cardiovascular events. The main conclusion of the study was that the presence of carotid artery plaque is an independent factor of the risk of cardiovascular events and that this type of screening could aid classic risk factors in the risk stratification of this population.
Patients with GH have traditionally been considered to be at high vascular risk because approximately 50% of men and 30% of women with untreated FH will experience a cardiovascular event before age 60 years.24 It had been estimated that in the 1970s, young adults with heterozygotic FH had a 100-fold increase in coronary disease mortality, which represented a life expectancy limited by 20 years in men and 12 years in women.25 Similar data have been reported for FCH18,26 and dysbetalipoproteinemia.27 However, statin therapy has been an advancement for persons with GH. Since the late 1980s, this pharmacological group has lowered cholesterol levels in these patients substantially and has changed the natural course of the disease. Two reports from the Simon Broome Registry in the United Kingdom and the Norwegian FH Registry indicate that ischemic heart disease mortality has fallen significantly in recent years, although it remains higher than in the general population.28,29
At present, all scientific societies recognize the value of statin therapy in GH; however, there are no uniform criteria to indicate the intensity, objectives, and indications of combined treatment with ezetimibe or novel PCSK9 inhibitors.10,23,30–32 The incorporation of novel treatments in GH cannot be assessed without understanding the absolute benefit of the intervention on lowering LDL-C levels.6,9 The relative benefit has been well established in meta-analyses of large statin studies, particularly those conducted by the Cholesterol Treatment Trialists Collaborators group.33 However, the absolute benefit (ie, the number of participants to be treated to prevent an event) will depend on the extent of the LDL-C reduction and the baseline cardiovascular risk of the participant.
There are currently no tools to calculate cardiovascular risk precisely in participants with GH, which also shows high variability.10 Several publications have reported on different risk factors associated with the presence of CVD in observational cross-sectional studies, mainly in the absence of statin therapy, and attempts have been made to define the most serious forms.34 However, there were no prospective studies to determine the risk of these patients, particularly those who were receiving statin therapy. Our study provides relevant information with important clinical implications: patients with GH and plaques have a 4-fold cardiovascular risk compared with participants who have no plaques, a risk that remains steady even when adjusting for the main risk factors. Our study points to a GH subgroup that accounts for a third of patients, but who account for more than 70% of the events that occurred, which means they will likely benefit from closer preventive intervention.
Our results in patients with GH, a population not previously investigated prospectively, confirm the association of the presence of carotid plaques seen in other populations. A meta-analysis of 11 population-based studies with more than 54 000 participants has shown that the presence of carotid plaque is a better predictor of cardiovascular events than IMT.15 Similar results were obtained in the BioImage study, which found that the presence of carotid plaque is independently associated with the appearance of cardiovascular events, particularly coronary events.35
The association between the presence of plaque and coronary events is evidence that arteriosclerosis is a systemic disease affecting a significant number of arteries in different organs.36 This is relevant in GH because the predisposition to CVD mainly affects coronary vessels, but also shows that coronary lesions are not an isolated phenomenon. Ten Kate et al.37 found an association between coronary lesions and carotid plaque, comparing coronary and subclinical carotid arteriosclerosis measured by carotid ultrasound and coronary angiography, respectively, in a group of 67 participants with FH, thus showing a strong association with the presence of plaques at the 2 sites.
Our study is an initial prospective approach for risk stratification in patients with GH. This is important because there are currently no stratification tools for this population. Likewise, the detection of subclinical arteriosclerosis is clearly shown to be a tool for the stratification of special populations, such as those with extreme risk factors.38
Limitations and StrengthsThe main limitation of our study was the lack of uniform treatment during patient follow-up, as our study population consisted of patients seen in regular clinical practice. Potential differences in prescribed therapy may have caused some of the associations found. However, this is unlikely because all patients were diagnosed and treated according to the same uniform protocol and received follow-up from the same medical team (F. Civeira and E. Jarauta) throughout follow-up. Our cohort was a young population with a mean age < 50 years attended in a specialized unit with strict targets for risk factor control. Extrapolation of our results to older populations in a different clinical setting might be inappropriate. The low number of events during follow-up also limited the soundness of the study.
The strong points of this study include the high number of patients, the long follow-up period, the strict criteria used for diagnosing GH and subclinical atherosclerosis and for therapeutic treatment based on guidelines established before follow-up, and the use of results from a single site, which limited clinical variability.
CONCLUSIONSIn summary, in patients with GH whose risk cannot be predicted with the equations used in the general population, ultrasound screening for carotid plaque identifies a patient subgroup with the majority of cardiovascular events. Our results support the use of subclinical arteriosclerosis screening and quantitation in this population and should aid risk stratification and therapeutic planning in patients with GH.
- –
Patients with GH are at high but variable cardiovascular risk.
- –
The risk equations used in the general population are not useful in GH.
- –
There are no reliable tools for risk stratification in GH.
- –
Novel cholesterol-lowering agents require careful selection of patients, based on their baseline risk.
- –
The presence of carotid plaque is associated with a 4-fold risk of CVD, compared with participants without plaque.
- –
The presence of carotid plaque can help ensure more accurate risk stratification in GH and more personalized treatment.
- –
The presence of carotid plaque raises risk in primary and secondary prevention.
This study received aid from the Ministry of Economy and Competitiveness of Spain: PI12/01087, PI12/01703, and PI12/01321 integrated in the National Plan of R&D +Innovation and co-funded by the Carlos III Health Institute, the European Fund for Regional Development, and the Cardiovascular Research Network RD12/0042/0055.
CONFLICTS OF INTERESTNone declared.