Keywords
INTRODUCTION
One of the most common--and most difficult--questions that arises when examining a contrast coronary angiography image is whether a lesion is significant or not, i.e., whether it could cause ischemia and therefore requires treatment. Doubts exist about the significance of up to 30% of the lesions for which angioplasty is performed.1 Frequently, observers within the same group have different opinions and consensus is difficult to attain, except in that the technique has limitations when assessing intermediate lesions (40%-70% stenosis by diameter). This review discusses the alternatives available in today's catheterization room for dealing with such uncertainties, including the advantages and limitations of different techniques.
THE ROLE AND LIMITATIONS OF CONTRAST ANGIOGRAPHY IN THE ASSESSMENT OF INTERMEDIATE LESIONS
Contrast angiography has been used to assess the severity of coronary lesions for nearly 5 decades. Forty-five years ago, Dr. F. Mason mistakenly injected contrast medium into the right coronary artery of a patient prepared to undergo aortography. At that time (1958) it was assumed that the immediate consequence of this--in agreement with observations in experimental models--would be malignant ventricular arrhythmias caused by transitory asymmetric hypoxia. The finding that such a selective injection could be performed reasonably safely provided an essential tool for the study of the natural history of heart disease, a tool still used today. It also provided a means of understanding the relationship between acute heart syndromes and the complications of atheroma plaques, and helped to introduce treatments such as fibrinolysis and percutaneous angioplasty.
The pioneering work of Gould et al2,3 allowed the description, in an experimental model, of the relationship between anatomical severity and stenosis, and the resistance this causes to the blood flow. In experimental models it is accepted that a reduction of more than 75% of the cross-section of a blood vessel (i.e., 50% stenosis by diameter) is necessary to reduce coronary blood flow to an extent capable of inducing ischemia during exercise. This has been extrapolated to clinical practice, where a stenosis of ≥50% is now generally accepted as being significant because of its theoretical potential to cause ischemia.
However, in recent decades contrast angiography has been found to have many limitations and the significance of lesions needs to be assessed. The visual estimation of stenosis severity lacks precision compared to automatic quantification techniques, especially with respect to intermediate lesions.4 The importance of lesions smaller than 40% tends to be underestimated, whereas that of lesions ≥50% is often overestimated. It is also difficult to distinguish between intermediate lesions able or unable to cause ischemia.5 Underestimation of the degree of heart disease has also been shown in comparison with histological results. This has been attributed to the disease generally being diffuse,6 and to the fact that angiography characteristically determines lesion severity through comparison with a presumed healthy section (in terms of percentage of stenosis by diameter). But if the reference segment is also diseased, the degree of stenosis produced by the lesion will, logically, be underestimated. This has been observed in vivo with intravascular echography,7 which has shown that at least 10% of the reference segments considered normal are not, and that on average, 50% of the lumen of these reference segments is occupied by plaque.
The majority of patients who undergo coronary angiography for the assessment of thoracic pain do so without having undergone any non-invasive test for ischemia.8 This makes it more difficult to evaluate the functional significance of lesions. Currently, this tendency has become more common, and it is likely to increase since present treatment guides for acute coronary syndromes favor early invasive management.9
The relative insensitivity of contrast angiography in detecting intermediate lesions capable of causing ischemia, as well as the number of patients who do not undergo non-invasive testing to determine whether ischemia is present, has led to increased interest in the development of new techniques to assess the physiological significance of intermediate lesions in the catheterization room. The three most commonly used methods are reviewed here: measurement of the coronary flow reserve (CFR) by Doppler guidewire, measurement of the myocardial fractional flow reserve (MFFR) by pressure guidewire, and intravascular echography. However, it should be pointed out that having complementary diagnostic techniques available does not mean that carefully performed angiography is any less important: along with clinical data, angiography is sufficient to assess many coronary lesions appropriately.
SOME PRINCIPLES OF CORONARY PHYSIOPATHOLOGY
The physiological basis for the use of flow and pressure sensors to assess lesions is simple. Resting coronary blood flow increases by several orders of magnitude in response to myocardial oxygen demand or drug stimulus. Under normal conditions, the greatest resistance to blood flow is produced by the precapillary arterioles; that induced by the epicardial arteries is negligible. A coronary stenosis is considered serious when it is able to reduce coronary blood flow and therefore induce ischemia during exercise. When stenosis increases the resistance to coronary blood flow in the conducting epicardial arteries, the microvasculature dilates to maintain a regional flow sufficient to cover the myocardial oxygen demand. These phenomena cause changes in blood flow and blood pressure.
Consequences for blood flow
Depending on the severity of a stenosis, the resting flow distal to the lesion can become reduced, although it is usually sufficient to cover the myocardium's basal metabolic needs. An increase in myocardial oxygen consumption in these circumstances, or artificial hyperemic stimulation, leads to an increase in distal blood flow that is less than would be expected for the region if there were no stenosis, or when compared to that of non-stenotic regions.
Consequences for blood pressure
An epicardial coronary stenosis capable of increasing resistance to blood flow causes a loss of distal pressure because of the reduction in kinetic energy through viscous friction, turbulence, and separation of the flow.10 As a consequence, a pressure difference, or gradient, develops between the areas before and after the stenotic region. This pressure gradient is directly related to the blood flow. In a situation of maximum hyperemia and maximum coronary arteriolar dilation, the relationship between blood pressure and flow is linear.
CORONARY FLOW RESERVE EVALUATED BY DOPPLER GUIDEWIRE
Concept
Coronary flow reserve is defined as the ratio between the coronary blood flow during maximum hyperemia and basal coronary blood flow. Assuming the cross-sectional area of the artery to be stable, the velocity of the blood is proportional to its flow, and therefore CFR can be estimated by measuring blood velocity at rest and during vasodilation with maximum hyperemia. In patients with coronary lesions, the CFR is >2 and can reach 5. In young patients with normal arteries (as determined by intravascular echography), CFR is usually >3, though in patients with chest pain and angiographically normal arteries it is usually somewhat lower, suggesting angiographically undetectable micro- or macrovascular disease.11
The technique and its clinical use
Intracoronary blood velocity is measured using a maneuverable guidewire similar to that used in angioplasty (0.014 inches, length 175 cm), with a piezoelectric transducer at its tip. This end of the guidewire is positioned distally to the stenosis, while the proximal part of the guidewire is connected to a console for signal analysis. The transducer transmits and receives ultrasound signals that bounce off the blood cells circulating in the artery; the difference in frequency between the transmission and return frequencies allows the blood flow velocity to be calculated according to the following expression:
V=(F1F0)x(C)/2F0)xcosxØ
where V is the velocity of the blood, F0 the transmission frequency, F1 the return frequency, C the constant for the velocity of sound in blood, and Ø the angle of incidence of the ultrasound waves.
Modern equipment performs spectral analysis of the images with fast Fourier transformation (Figure 1), and the console either measures or automatically calculates the different blood velocity variables. Coronary flow reserve is the ratio of the blood flow at maximum vasodilation (after administering a vasodilator) to the basal blood flow. The vasodilator most commonly used is adenosine, which is administered as an intracoronary bolus or as a continuous intravenous infusion. Other pharmacological agents, such as papaverine, were once much used but are now employed only rarely; the use of papaverine decreased because it can cause ventricular arrhythmias. Intracoronary nitroglycerin should be administered to normalize arterial tone before velocity measurements are made. It is assumed that the cross-sectional area of the vessel does not vary substantially either at rest or with the induction of maximum hyperemia, making it possible to calculate the coronary flow (mL/s) by multiplying blood velocity (cm/s) by cross-sectional area of the artery (cm2).
Fig. 1. Coronary flow velocities recorded by Doppler guidewire. The upper panels of each figure (A and B) correspond to velocity in real time. The lower left panels of each figure show the baseline velocity, and the lower right panels show the velocity recorded after maximum hyperemia was induced with adenosine. The ratio of the average peak velocity (APV) in hyperemia to baseline velocity is the coronary flow reserve (CFR). In A, the CFR is 2, and in B it is 1.3, indicating a severe lesion or microvascular damage.
Other Doppler variables have been used to assess the severity of epicardial lesions, such as the ratio of the proximal to distal velocity (P/D), or the diastolic/systolic velocity ratio (DSVR). In the coronary arteries, the volume of blood flow and the cross-sectional area of the artery become proportionally less along the length of the vessel. Therefore, for arteries with a diameter of >2 mm, the velocity in the distal part of the vessel is similar to that at the proximal end, and the P/D ratio is close to 1. In significant stenosis, a P/D ratio of >1.7 is seen.12 In addition, the normal predominance of the diastolic flow is altered with respect to the systolic flow, and the DSVR, which is normally >2, decreases to near 1.13 Unfortunately, both the P/D and the DSVR show variability and uncertain specificity. They are therefore little used in clinical practice.14
Limitations
By definition, the circumstances that alter basal coronary flow or reduce the maximum degree of hyperemia must also alter CFR. A characteristic of CFR is its dependence on hemodynamic conditions. For example, tachycardia increases basal blood flow,15 and should therefore be taken into account in serial studies. In addition, factors that increase oxygen consumption, such as anemia or hyperthyroidism, or changes in load or contractility conditions, can also alter basal flow rate. Wieneke et al16 have proposed a correction factor derived from basal blood flow and the age of the patients, to provide the corrected CFR.
Coronary flow reserve jointly evaluates epicardial and microvascular epicardial components. A CFR of >2 indicates that both components are normal, but stenosis in the epicardial arteries or microvascular dysfunction can alter the flow reserve. Abnormal CFRs have been described in patients with essential hypertension or aortic stenosis but with angiographically normal epicardial arteries, as well as in patients with diabetes and in situations of chronic and acute ischemia.18 Similarly, after an acute myocardial infarction, microvascular damage can cause a decrease in CFR, regardless of the existence of epicardial stenosis.19 To try to separate the epicardial and microvascular components, and to assess the severity of epicardial stenosis more directly and without confounding factors, the use of relative CFR (rCFR) has been suggested. Relative CFR is the ratio between CFR in the stenotic artery to be evaluated and CFR of a reference, non-stenotic artery. Assuming that the response of the microvasculature to hyperemic stimulus is similar in both, the ratio will reflect the repercussion of the epicardial lesion on coronary blood flow. The normal value for rCFR is between 0.75 and 1.20,21 Logically, rCFR cannot be used in patients with 3-vessel disease since no reference value can be obtained. Neither can it be used in patients in whom the microvascular response may not be homogeneous across the myocardium, such as in those with segmental ventricular dysfunction or those who have suffered a myocardial infarction.
Validation for the assessment of intermediate lesions (Table 1)
Several studies have evaluated the usefulness of measuring CFR by Doppler analysis to assess intermediate lesions. In a pioneering study now more than a decade old, Miller et al22 compared CFR with radioisotope perfusion techniques in 27 patients with intermediate lesions (30%-70% stenosis by diameter). The 14 patients with a CFR of <2 as determined by Doppler analysis were found to have reversible ischemia in the radioisotope study, whereas 10 of the 13 patients with a CFR >2 were found to be normal. The agreement between the 2 techniques was 89%. Joye et al,23 who examined 30 patients with intermediate lesions, also found an excellent correlation between the 2 techniques, with a sensitivity and specificity of 94% and 95% respectively. Unfortunately, in later studies performed with larger cohorts of patients, comparison with radioisotope perfusion or stress echocardiography24-26,21 has shown less agreement with CFR results (72%-84%).
As mentioned above, the value of rCFR has also been studied in an attempt to eliminate the dependence of CFR on the microvascular response. Baumgart et al27 used ultrasound to study the relationship between CFR, rCFR, MFFR (see below) and the percentage stenosis per area in 24 patients. The MFFR and rCFR correlated well with percentage stenosis per area (r=0.89 and r=0.79 respectively; P<.0001). Similarly, a good correlation was found between MFFR and rCFR (r=0.91; P<.0001), but not with absolute CFR (r=0.33; P=NS). This underscores the substantial dependence of this variable on the microvasculature, as well as the greater potential use of rCFR to assess epicardial lesions. More recent studies, such as that of Chamuleau et al,28 report a poorer agreement between the results of perfusion tests with MIBI-dipyridamole and CFR and rCFR in patients with 2-vessel disease (76% and 78% respectively), although radioisotope techniques are known to be less precise for the location of lesions in multivessel disease.29
MYOCARDIAL FRACTIONAL FLOW RESERVE EVALUATED BY PRESSURE GUIDEWIRE
Concept
Myocardial fractional flow reserve is defined as the ratio between maximum coronary flow and the myocardium in the presence of a stenosis, divided by the maximum coronary flow that would exist in the same vessel if there were no stenosis.30,31 In other words, it is the fraction of maximum coronary flow capable of being transported by the stenotic vessel. In maximum hyperemia with maximum coronary arterial vasodilation, the relationship between pressure and coronary flow is linear, which allows the MFFR to be calculated. In clinical practice, this involves simply dividing the mean pressure distal of the stenosis by the mean aortic pressure under conditions of pharmacologically-induced maximum hyperemia. In normal arteries with no stenosis, and therefore with no reduction in pressure, the MFFR is, of course, equal to 1. Some authors have suggested calculating MFFR only with the gradient produced during systole, given that the greater part of coronary blood flow occurs during this phase of the cycle. However, the clinical superiority of this approach over conventional MFFR has not been demonstrated.32
The technique and its clinical use
For years, pressure gradients caused by stenosis have been measured in the catheterization room. They were widely used to evaluate the significance of intermediate lesions, as well as the results of balloon angioplasty.33,34 Later, they were abandoned because the angioplasty catheters used to measure the gradients were themselves a certain obstacle to blood flow. Moreover, it was still unknown that the relationship between pressure and flow was most significant only in maximum hyperemia (and not at rest), since the pressure gradient is, of course, linked to flow. Currently, 0.014-inch guidewires of similar appearance and maneuverability to those used in conventional angioplasty are used to calculate MFFR. The guidewire incorporates a pressure transducer close to its tip, and this is introduced distally to the lesion under study. The proximal part of the guidewire connects to a console for signal analysis. After baseline pressures are recorded, a hyperemic stimulus is provided to achieve maximum vasodilation. The MFFR is calculated as the ratio between the pressure distal to the stenosis (measured by the pressure guidewire) and the pressure proximal to the lesion (measured with the guide catheter) (Figure 2). It is accepted that a lesion is capable of causing ischemia when the MFFR is <0.75.35,36 In addition, measurement of the pressure distal to the inflated balloon (for example during an interventional procedure) reflects the wedge pressure at that site, allowing the collateral circulation to be evaluated.
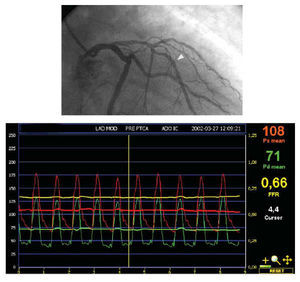
Fig. 2. Anterior right oblique projection showing an intermediate lesion in the middle third of the anterior descending coronary artery, distal to a well developed septal branch. There is also another mild lesion close to the septal branch. After positioning the transducer of the pressure guidewire distal to the stenosis (arrowhead) and inducing maximum hyperemia with intravenous adenosine, a mean pressure of 71 mm Hg was recorded by the pressure guidewire transducer and 108 mm Hg by the catheter guidewire. The ratio of the two values for myocardial fractional flow reserve (MFFR) was 0.66, indicating a significant lesion.
It is also possible to assess multiple sequential stenoses. The MFFR calculated with the mean pressure distal to the most distal stenosis reflects how all the lesions together compromise the blood flow. The contribution of each to the reduction in MFFR can also be calculated separately, although this is considerably more complicated and requires occluding the artery to determine the wedge pressure at that site.37,38 Finally, it is also possible to assess the significance of a diffusely diseased segment with no localized lesion that appears obviously to be severe on angiography. Maximum hyperemia is maintained by an intravenous infusion of adenosine while the transducer is carefully withdrawn over the length of the segment. An MFFR of <0.75 in the most distal part of the artery, and decreasing gradually along its length, shows the stenosis to be severe. It also shows there is no localized lesion that might benefit from percutaneous dilatation.
In summary, the pressure guidewire provides a great deal of information on the pathophysiology of the coronary artery under study, and its use has been validated for many, commonly-faced clinical situations.
Limitations
Calculation of MFFR is based on the assumption that the relationship between pressure and coronary flow is linear when hyperemia is maximal. If maximum hyperemia is not reached, the pressure gradient will be underestimated and the MFFR overestimated--and therefore a physiologically significant lesion might be considered non-significant. The limit of 0.75 was validated by de Bruyne and Pijls with the use of intracoronary papaverine and intravenous adenosine in selected, stable patients with single-vessel disease and normal left ventricular function.35,36,39 However, most catheterization rooms use intracoronary adenosine as the vasodilation stimulant (administered via a guide catheter) owing to its ease of use and rapid action, although the dose required to induce maximum vasodilation is yet to be definitively established.40 One of the advantages of this technique is that there is a categorical limit: beyond 0.75 a lesion is considered capable of causing ischemia. Nevertheless, as always occurs in biology, this limit is probably too rigid, and nowadays a more flexible range of 0.75-0.80 is gaining acceptance.
A final limitation is that maximum hyperemia cannot be attained when there is microvascular dysfunction, as occurs after an infarction, in patients with diabetes, or in those with left ventricular hypertrophy. In these cases MFFR will therefore be overestimated. However, it has been noted that these vessels will probably not benefit from revascularization, because their lesions do not lead to ischemia even when vasodilation is at its maximum.
Validation for the assessment of intermediate lesions (Table 2)
The correlation between the MFFR and non-invasive tests for the detection of ischemia in intermediate lesions has been the subject of careful study.28,32,35,36,41 In their pioneering work, Pijls et al36 measured the MFFR in 45 patients with intermediate coronary stenosis, who underwent an exercise test, a coronary perfusion test with isotopes, and stress echocardiography. All 21 patients with an MFFR of <0.75 showed evidence of ischemia in at least one of the non-invasive tests. In 21 of the 24 who had MFFR ≥0.75, ischemia was induced in none of the tests. The sensitivity, specificity and agreement of MFFR was 88%, 100% and 93% respectively. On the basis of these results, a value of 0.75 was established as the limit for considering a lesion capable of causing ischemia. Similar results have been obtained in more recent studies with other patients (sensitivities around 90%, agreement >90%).32,41
It should be pointed out that some data support the idea that it is safe not to undertake intervention in lesions with MFFR>0.75.42-47 Bech et al46 measured MFFR in 325 patients referred for angioplasty but with no evidence of ischemia. Those with MFFR>0.75 were randomized to receive (n=90) or not receive (n=91) angioplasty (the 144 with MFFR<0.75 all received angioplasty). Event-free survival was similar in both groups (92% compared to 89% at 12 months, and 89% compared to 83% at 24 months), and significantly better than in the group with MFFR <0.75 (patients who underwent angioplasty directly). These results suggest that calculation of MFFR helps to identify patients who will benefit from vascularization.
COMBINED USE OF PRESSURE GRADIENT AND CORONARY FLOW TO PREDICT LESION SEVERITY
It has recently been suggested that the combination of pressure and blood flow or blood velocity data (measured using pressure and Doppler guidewires) might provide better information than any of these techniques on their own. Meuwissen et al48 reported, after performing perfusion tests with radioisotopes in 151 patients with angina, that the best predictor of a reversible perfusion defect was the index of resistance of the stenosis during hyperemia. The curvilinear relationship between flow and pressure gradient is well known, and prediction of severity of a lesion based on both variables can logically be expected to be more precise than prediction based on one variable alone. This index normalizes pressure gradient by blood velocity or coronary flow, and can therefore resolve some of the conditions in which coronary flow is inadequately high (a false positive MFFR) or low (false negative MFFR). In the near future it is foreseeable that velocity and pressure transducers mounted on the same guidewire will become available, making their everyday clinical use practical. Pressure guides capable of measuring temperature have been developed, and can be used to measure coronary flow by thermodilution (although they are not yet commercially available).49
EVALUATION BY INTRACORONARY ECHOGRAPHY
The many limitations of contrast angiography, the fact that it cannot show the atheroma plaque directly, plus the two-dimensional representation of the lumen that it provides, have spurred the development of alternative imaging systems such as intracoronary ultrasound.
The development of these systems has been possible thanks to transducer miniaturization to <1 mm in diameter, a size that allows them to pass readily through the coronary arteries. The principles by which images are obtained are the same as for any other ultrasonic imaging system. Ultrasound is generated by one or more l small transducers which scan the vessel circumference, producing a tomographic image of the artery in cross-section. Some of the transmitted waves are reflected from the artery wall back towards the transducer, mainly from interfaces of tissues with different acoustic impedances. The magnitude of the reflected ultrasounds depends on the difference in the acoustic impedance of the 2 adjacent tissues. The greater the echogenicity, the greater the capacity to reflect ultrasound, and therefore the more intense the signals in the final image. The high frequency at which intracoronary transducers operate (30-40 MHz) provides excellent spatial resolution, i.e., considerable power to discriminate between objects very close to one another in the image. Axial spatial resolution, i.e., in the direction of the ultrasound beam, is on the order of 80-150 micrometers, and lateral resolution, i.e., perpendicular to the ultrasound beam and the catheter, is 200-250 micrometer.50,51 It is thus possible to obtain very detailed images of the lumen and arterial walls or the lesion being studied. A certain degree of tissue characterizatie. The tomographic assessment of this technique allows a much more detailed analysis of the severity of the lesion (Figure 3), and of the existence of diffuse disease--one of the most common causes of underestimation of lesions examined by angiography.
Fig. 3. Despite multiple angiographic projections, the significance of the lesion in the first marginal branch was uncertain. However, in the intravascular echography study (B) the transducer (*) was almost completely surrounded by the atheroma plaque, and there was only a small residual volume at 5 o'clock (arrowhead), indicating a significant lesion. Note a degree of diffuse atheromatosis affecting the segments proximal (A) and distal (C) to the stenosis (arrowhead).
The technique
Two commercial ultrasound systems are available for intracoronary use: mechanical and solid state. In the mechanical system, a single transducer rotates at 1800 rpm (via an external cable), scanning the surface of the vessel. The transducer lies within a protective sheath that prevents it from contacting the arterial wall. In solid state systems, many small transducers (up to 64 in modern systems) are mounted around the catheter and each one scans a sector of the arterial circumference. The catheter is introduced into the coronary arteries threaded onto an angioplasty guidewire in a fashion analogous to that of a balloon catheter, and the lesion is scanned during manual or automatic withdrawal at constant speed. As with pressure and Doppler guidewires, the patient requires anticoagulation treatment with 5000-10 000 U heparin, and intracoronary nitroglycerin should be used systematically to avoid arterial spasm. The images obtained are stored digitally or on videotape for later analysis. The better definition of the anatomical information provided by ultrasound allows one to understand and interpret many images that are ambiguous in angiographic studies.
Limitations
Ultrasound provides much better anatomical information than contrast angiography, but it offers no functional information on the severity of lesions. Therefore, its main limitation is that any assessment of the severity of a lesion is an extrapolation of its anatomical characteristics: severity is not measured directly. There is also the inherent risk surrounding the introduction of instruments into the coronary vessel. However, the risk of adverse events is small--less than 0.3% for major complications--and is mainly related to the assessment of severe lesions during intervention procedures.52,53
Validation for the assessment of intermediate lesions (Table 3)
A number of studies have tried to determine a cut-off point that can classify a lesion as significant and potentially capable of causing ischemia. These studies have tried to validate ultrasound techniques, comparing them with reference patterns in different invasive and non-invasive techniques. Abizaid et al54 studied the relationship between the minimum lumen area evaluated by echography and CFR evaluated by Doppler analysis in 73 patients. These authors found that a minimum lumen area of <4 mm2 showed 89% concordance with a CFR of <2. Nishioka et al55 compared the findings of intravascular echography with those of myocardial perfusion with radioisotopes in 70 patients, most of whom had intermediate lesions. Again, a minimum lumen area of ≤4 mm2 by echography showed a sensitivity of 88% and a specificity of 90% in predicting perfusion defects in radioisotope images. Other ultrasound variables such as plaque load, i.e., the fraction of the area of the vessel occupied by the plaque, provided somewhat lower sensitivity and specificity, although still in the 80-90% range.
The relationship between echocardiographic variables and MFFR measured by pressure guidewire has also been investigated. In 51 lesions (half of which were angiographically intermediate), Takagi et al56 found MFFR to correlate well with minimum lumen area (r=0.79; P<.0001) and plaque load (r=0.77; P<.0001). With a cut-off value of 3 mm2 minimum lumen area, the sensitivity of ultrasound images in detecting MFFR<0.75 was 83%, and specificity 92%. Similarly, with a plaque load of >60% to indicate significance, the sensitivity of ultrasound was 92% in detecting lesions significant according to MFFR, and specificity was 89%. In a more recent study, however, Briguori et al57 reported lower levels of agreement. These authors studied 53 lesions, all intermediate, and found a minimum lumen area of ≤4 mm2 had a sensitivity of 92% in detecting lesions considered significant according to the MFFR results. Specificity was, however, only 56%. The criterion of >70% plaque load provided 100% sensitivity, but only 68% specificity. These authors propose the combined use of >70% plaque load and minimum lumen diameter of ≤1.8 mm, which in their study provided 100% sensitivity and 76% specificity.
The usefulness of some of these criteria has been assessed, not for predicting ischemia in functional tests but for predicting clinically adverse events during follow-up. Abizaid et al58 indicate that a minimum lumen area of ≥4 mm2 or a minimum lumen diameter of ≥2 mm predicts a low incidence of death or myocardial infarction--only 2% in the following 13 months. These authors suggest that by using these criteria, intervention can be avoided and good clinical results obtained.
WHEN TO USE ONE TECHNIQUE OR ANOTHER? ADVANTAGES AND DISADVANTAGES (TABLE 4)
The use of CFR has several limitations. This variable is dependent on hemodynamic conditions, and therefore potential reproduci bility is diminished. Further, although a value above 2 is considered normal, this is no clear cut-off point--this varies from patient to patient. The Doppler guidewire must be positioned with utmost care, because centering the transducer in the artery is essential to obtain a good signal. Finally, CFR simultaneously evaluates the epicardial coronary component as well as the microvascular component. Therefore, patients with microvascular alterations (e.g., because of myocardial hypertrophy or diabetes) may have an abnormal CFR yet have no significant epicardial lesions. If disease is diffuse, rCFR can be used to determine the severity of epicardial stenosis, but this cannot, of course, be used in patients with three-vessel disease. A vessel other than the one under study must always be available to receive the catheter.
Coronary flow reserve can only be adequately determined by Doppler analysis, and MFFR can only be adequately determined by pressure guidewire, if maximum hyperemia is achieved. If it cannot be achieved, the pressure gradient across the stenosis will be underestimated, as will CFR, and MFFR will be overestimated. Maximum vasodilation is therefore critical. Unfortunately, in clinical practice most studies have been done with intracoronary adenosine because of its ease of use and rapid action, rather than with intravenous adenosine--the method with which the technique was validated. Further, the ideal dose for intracoronary administration is still under discussion: those currently used are probably well below what is needed to induce maximum dilation.40 Myocardial fractional flow reserve is also affected by the presence of microvascular disease, because of the need to obtain maximum hyperemia. If there is microvascular damage, MFFR will be overestimated. However, it can still provide useful information since a value of >0.75 implies the absence of ischemia in the myocardium. This indicator has the enormous advantage of having a more categorical limit for normality (1) and for the induction of ischemia (0.75). It can be measured in patients with multivessel disease, and takes into account the presence of collateral circulation. However, its use is not validated, and it is reasonable to expect it to be less useful for lesions that cause unstable syndromes, in which dynamic factors such as thrombosis or changes in arterial tone play an important role. The main limitation of intracoronary echocardiography is that it provides only anatomical information, and the functional significance of the lesion must be deduced from these findings. But the correlation is not always good, and there is no universally useful cut-off point. However, it has several advantages: it provides excellent tomographic anatomical detail, it can resolve many ambiguous angiography images, it facilitates our understanding of the causes of the problem, and it can even help determine whether interventional treatment is necessary. Finally, the interpretation of the images, especially in uncertain cases, requires a degree of knowledge of the technique that represents a barrier to some specialists in hemodynamics.
Operator experience with the different techniques is important when deciding which to use to evaluate an intermediate lesion. All should be used meticulously if reliable information is to be obtained, and none are complication-free (although the incidence of adverse events is low). Considering all the above, the pressure guidewire is, at the present time, generally the most useful instrument for evaluating lesions of uncertain significance in the context of stable lesions (Figure 4). In fact, although still limited, its use has increased considerably in Spain in the last few years.59 When knowledge of the anatomy or the composition of a lesion can be of help in planning an intervention, intravascular echography may be a good alternative if the operator has sufficient experience.
Fig. 4. Figure legend translation pending
It should be pointed out that the use of intracoronary diagnostic methods to evaluate angiographically uncertain lesions is still low in Spain.59 This is because of the extra time they require, the additional costs, sometimes because of a lack of operator experience, and because of the current ease with which these lesions can be treated. In fact, they are frequently treated in less time than it takes to assess them, and the decision to check their clinical repercussions in the patient is often deferred in the confidence that they can be adequately managed should the need arise. Given the growing epidemic of patients who arrive in the catheterization room without having undergone non-invasive testing to detect ischemia, the moment may have arrived when familiarity with at least one of these techniques is necessary.
This section is sponsored by Laboratorio Dr. Esteve
Correspondence: Dr. J. Botas.
Servicio de Cardiología. Hospital General Gregorio Marañón.
Dr. Esquerdo, 47. 28007 Madrid. España.
E-mail: javbotas@jet.es