Keywords
INTRODUCTION
The aim of our study was to review the role of various imaging techniques used for diagnosing, evaluating, and making decisions for patients with cardiac tamponade or restrictive pericarditis. It is indisputable that the use of echocardiography, computerized tomography (CT), and magnetic resonance (MR) for diagnosing and treating diseases of the pericardium has been vital; currently, to be deprived of these techniques in daily practice would be difficult. On the other hand, it is evident that these imaging techniques must be used in a manner that is complementary to clinical evaluation, and not as a substitute for it. Clinical evaluation based on history, detailed physical examination, epidemiological data, and the interpretation of diagnostic and basic tests, such as chest radiograph and electrocardiography, must be the initial core and essential approach when treating patients with pericardial disease. Therefore, although in this study we reviewed the possible results that these imaging techniques may provide, the basic aim of the study was to integrate those findings into the clinical evaluation process, and to discuss the specific role of these imaging techniques in diagnosing and treating patients.
CARDIAC TAMPONADE
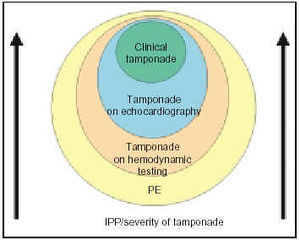
Before describing the findings of the various imaging techniques, the concept of cardiac tamponade should be discussed briefly since it is important in interpreting results and in the decision-making process. Until recently, it was accepted that cardiac tamponade was an «all or nothing» situation; in other words, that patients either had or did not have cardiac blockage. This belief was rooted in experimental studies, particularly in a study by Holt1 on the pericardial sac of dogs (after extraction of the heart and large vessels), in which it was shown that injecting progressive amounts of fluid was not accompanied by an increase in intrapericardial pressure until it reached a critical volume, at which point the intrapericardial pressure was subject to a sudden influx and increased very rapidly. From the clinical standpoint, this model is probably correct and applicable in cases of acute cardiac tamponade, such as cases accompanied by cardiac rupture or traumatic tamponade. In the majority of cases, however, of cardiac tamponade resulting from various medical conditions, the pericardial liquid accumulates in a much more gradual manner, setting into motion compensatory hemodynamic mechanisms; as a result, signs of blockage appear in a more insidious and progressive manner. Therefore, in most patients with pericardial effusion, the volume-to-pressure curve has a lesser gradient, and produces a much more progressive and continuous change in hemodynamic parameters. This phenomenon has been shown in experimental studies of animal models with in situ hearts2 and in clinical studies. Reddy3 elegantly developed the concept of tamponade as a continuum after studying 77 patients with pericardial effusion who underwent pericardiocentesis with monitoring of intrapericardial, right atrial, and pulmonary capillary pressure. This study showed changes in arterial pressure, cardiac output, and variations in arterial pressure with inspiration, as well as slight increases in intrapericardial pressure (inferior to the pressure of the right atrium), which reversed after pericardiocentesis was performed. In light of these observations, the authors concluded that there is no effusion that does not cause hemodynamic changes and that, therefore, all patients with effusion suffer blockage. More than the presence or absence of effusion, what the clinician needs to evaluate is the severity of effusion; this view is consistent with our experience with asymptomatic patients with chronic pericardial effusion with no signs of tamponade, in whom intrapericardial pressure is consistently found to be increased and transmural pressure of the right ventricle reduced, anomalies that normalize after performing pericardiocentesis.4 Another important point is that although increased intrapericardial pressure is the determining factor in tamponade, both its clinical and hemodynamic manifestations, such as those seen on echo-Doppler, do not depend exclusively on it. The state of volemia, previous intracardiac pressure, and the thickness and rigidity of the cardiac walls can change the appearance of signs of blockage in one way or another; the appearance of collapse on echocardiogram is particularly influenced by these factors.
Therefore, the concept of tamponade being a continuum and not an all or nothing situation that is also influenced by independent intrapericardial pressure variables is important when establishing clinical-to-echocardiographic-to-hemodynamic correlations, and when making decisions about diagnostic or therapeutic procedures, as we will expound upon later.
Doppler echocardiography
The most characteristic signs of tamponade on echo-Doppler are listed in Table 1. The reciprocal respiratory changes in ventricular function (eg, an exaggerated increase in the right ventricle diameter with a decrease in the left ventricle diameter during inspiration, with the opposite occurring during expiration), shows the competitive filling of both ventricles within a reduced pericardial space. This sign, which can be seen both on M-mode echocardiogram and 2-dimensional echocardiogram, is perhaps one of the most specific signs of tamponade,5 probably because it occurs only in cases of serious tamponade. In patients with chronic obstructive pulmonary disease there may also be important changes in ventricular diameter during the respiratory cycle.
The telediastolic collapse of the right atrium6 and the diastolic collapse of the right ventricle7 (Figure 1) are probably the most well-known signs on echocardiography, and are the signs that occur most frequently in cardiac tamponade. These signs are better visualized on 2-dimensional echocardiogram with 4-chamber apical and subcostal projections.
Fig. 1. 2-dimensional echocardiogram (4-chamber apical plane) showing a significant atrial (small arrows) and right ventricle (large arrows) collapse. The patient showed clinical signs of blockage. The collapse of both cavities is highly specific and has a high positive predictive value for tamponade. RA indicates right atrium; PE, pericardial effusion; RV, right ventricle; LV, left ventricle.
The collapse of the right atrium is considered to be an important indicative signs (it is present in nearly 100% of patients with cardiac tamponade in some series6) as it shows the presence of intrapericardial pressure that is higher than the right atrium telediastolic pressure, which is the moment that the volume of the right ventricle is the highest. Nevertheless, the severity of the collapse is not shown to be strictly related to intrapericardial pressure, which may be due to the limitations of echocardiography or to the fact that changes in volemia or intracavity pressure can augment or attenuate the appearance of the collapse. Subsequently, for example, the collapse of the right atrium can be absent or attenuated in patients with an increase in right atrial pressure due to another cause, such as tricuspid insufficiency or arterial pulmonary hypertension.7 In these cases, the isolated collapse of the left atrium can be seen.
We studied the correlation between the clinical and echocardiographic findings in 1 prospective series of 110 patients with moderate or severe pericardial effusion, of whom 38 had clinical signs of tamponade.8 The most important findings in this study were as follows; first, the collapse of the right atrium was present in 53% of the patients in the series. Second, the majority of patients (89%) with clinical signs of tamponade had collapse of at least 1 cavity (the collapse of the right atrium was the most common); however, we did not observe collapses in 4 of the 38 patients with clinical tamponade. Third, we observed collapse of the right atrium in 33% in patients with no clinical signs of tamponade, while collapse of the right ventricle occurred less frequently (in 10% of patients); the simultaneous collapse of both cavities occurred in 8% of patients. Therefore, the absence of collapse correlates well with the absence of tamponade, while the presence of collapse, especially of the right atrium, correlates poorly with the clinical signs of tamponade. The reason that some patients with evidence of clinical tamponade (evident on manometric recording during the pericardiocentesis) do present with collapses may be related to the limitations of the technique in the presence of adhesions between the pericardium and the cardiac walls, or due to the fact that in some patients the tamponade may cause compression of the vena cava or the pulmonary veins.9 The sensitivity, specificity, positive predictive, and negative predictive values that we found in our study are shown in Table 2. It should be mentioned that the predictive value of the collapses varied according to the prevalence of the illness (tamponade) in the population studied; in other words, according to the pretest probability of the disease occurring.10 Therefore, in the patients with a high clinical likelihood of having tamponade (jugular ingurgitation, paradoxical pulse), the presence of collapse visualized on echocardiogram practically ensured the diagnosis of tamponade. On the other hand, in patients without clinical suspicion of tamponade, the presence of collapse had a very low positive predictive value for tamponade, while the absence of collapse completely ruled it out.
The frequent finding of collapse, especially of the right atrium, in patients without clinical signs of tamponade indicates the presence of an increase in intrapericardial pressure that at some point in the cardiac cycle exceeds the intracavity pressure, but that is not severe enough to cause clinical tamponade. There has been discussion as to whether these patients should be categorized as having tamponade, and whether, in effect, tamponade should be a clinical or echocardiographic diagnosis.11,12 Actually, this observation supports the continuum concept of tamponade, as is shown in Figure 2. In the majority of patients with pericardial effusion, an increase in intrapericardial pressure with a consequent decrease in transmural pressure can be documented; this is corrected with pericardiocentesis. As the intrapericardial pressure increases, echocardiographic signs of tamponade appear and, finally, clinical signs appear, which indicate a higher level of severity in the setting of tamponade.
Fig. 2. Graphic showing the continuum concept applied to cardiac tamponade. Practically all pericardial effusions cause an increase in intrapericardial pressure and a certain degree of tamponade. Initially the disturbance can only be detected by the recording of intrapericardial and intracavity pressures. As intrapericardial pressure and severity of the tamponade increase, echocardiographic signs appear, and, ultimately, the clinical signs of tamponade appear. PE indicates pericardial effusion; IPP, intrapericardial pressure.
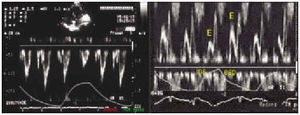
Among the possible signs shown on echo-Doppler, the most useful for diagnosing tamponade is an exaggerated decrease in mitral and aortic fluid with respiration (Figure 3), while the anomalies in the inferior vena cava and the suprahepatic veins are more difficult to identify technically8 and, possibly, are also more difficult to interpret.
Fig. 3. Aortic (left panel) and mitral (right panel) fluid in a patient with cardiac tamponade. The marked variation of the signal (E) can be seen with the clear signal decrease during inspiration. The marked respiratory variation in the aortic fluid is a less frequent finding, but it is very specific for tamponade, occurring only in serious cases. Ins indicates inspiration; exp, expiration.
All things considered, the most important contribution of echo-Doppler in patients with suspected tamponade is its ability to document the presence of significant pericardial effusion. In addition, echo-Doppler can provide findings that suggest increased intrapericardial pressure and can facilitate the evaluation of the severity of hemodynamic repercussions. Nevertheless, all such data must be considered in combination with data from the clinical evaluation in order to achieve an accurate, comprehensive interpretation and to facilitate appropriate decision-making.
Indications of pericardial effusion (pericardiocentesis or surgical drainage) (Figure 4)
Fig. 4. This figure is shows a graphic of moderate and severe pericardial effusion treatment, as discussed in the text. Note that once the presence of significant pericardial effusion has been documented, the treatment of these patients is primarily based on clinical criteria. The remaining data obtained from echo-Doppler (collapses, venous fluids) are always useful, but probably are relevant only in patients who present with hemodynamic disturbances that are difficult to evaluate. AIP indicates acute idiopathic pericarditis; CPE, chronic pericardial effusion.
The presence of pericardial effusion is very evident in patients who present with clinical signs of severe tamponade with jugular ingurgitation, paradoxical jugular ingurgitation, paradoxical arterial pulse, and arterial hypotension. In these cases, the fundamental value of the echocardiogram is that it proves the presence of significant pericardial effusion, and the almost certain finding of collapse in the cavities and anomalies in the mitral fluid. It must be taken into account that in patients with acute cardiac tamponade, such those with cardiac rupture from acute myocardial infarction, the amount of effusion may be quite small and the remaining echocardiographic signs may be difficult to visualize due to the critical nature of the patient´s condition. In such cases, clinical suspicion of the disorder is essential. Another sign established by pericardiocentesis is suspected purulent pericarditis;13 this procedure must be performed in patients with intrathoracic or subphrenic bacterial infections or sepsis when moderate or severe pericardial effusion it evident on echocardiography. In these cases, pericardiocentesis must be performed independently of clinical findings of hemodynamic compromise and regardless of whether there is evidence of collapse on echocardiogram.
In the other cases, the indication for pericardiocentesis is more evident on a case-by-case basis, and must be based on evaluation that combines the clinical data (including the epidemiological aspect of etiologic sources) and the echocardiographic findings. A frequently occurring presentation is that of young patients with acute inflammatory pericarditis, that, given its frequency, is considered to have (in all probability) a viral or idiopathic etiology, and which presents with a mild tamponade, with moderate or significant pericardial effusion, and collapse of the right cavities. Many of these patients have a good outcome with anti-inflammatory treatment, enabling postponement of performing peric ardiocentesis until the tamponade becomes severe. Treatment would be different in patients who had the same hemodynamic and echocardiographic features but in whom, for other reasons, there was a reasonable suspicion of a specific etiology (particularly tuberculosis), or in those patients for whom an unfavorable hemodynamic course was predictable due to the presence of neoplastic pericarditis or uremic pericarditis requiring dialysis.
There is no agreement on the indication for pericardial drainage in patients with significant pericardial effusion who do not present clinical signs of tamponade. Some authors14,15 recommend systematic drainage, citing diagnostic and therapeutic benefits; however, many of theses diagnostic procedures were unnecessary or the diagnosis could have been established by noninvasive procedures. In our experience16 in a series of 71 patients with a severe pericardial effusion (more than 20 mm) of various etiologies (acute pericarditis, systemic diseases) with no clinical signs of tamponade, pericardial drainage (pericardiocentesis or subxiphoid surgical drainage) had a very low diagnostic yield (7%) in the 26 patients in whom it was performed. On the other hand, of the 45 patients treated conservatively, none developed tamponade, and no new diagnostic tests were performed at followup. This even held true for the 25 patients who had collapses that were visible on echocardiogram. Therefore, we believe that pericardial drainage is not routinely indicated for patients with significant pericardial effusion who have no clinical signs of tamponade even in the presence of apparent collapses on echocardiogram. An exception would be those patients with chronic (proven to be present for more than 3 months) or severe (more than 20 mm) pericardial effusion, probably idiopathic; these patients run the risk of developing tamponade; additionally, pericardiocentesis can totally or partially resolve the effusion in approximately one-quarter of these patients.4
Transesophageal echocardiogram may be useful for patients who are poor candidates for echocardiography and in some patients with localized pericardial effusion. Transesophageal echocardiogram is particularly useful for evaluating patients who present with a poor hemodynamic course postcardiac surgery, and allows detection of the presence of hematomas that cause localized compression on a cardiac cavity, particularly the atria (Figure 5).17-19 Aside from these types of patients, transesophageal echocardiogram should not be considered to be a routine technique for the detection and treatment of pericardial effusion.
Fig. 5. The figure corresponds to a patient who presented with serious hemodynamic disturbance postoperatively following aortocoronary graft. On transesophageal echocardiogram, a collection of high-density fluid (arrow) can be seen which compressed and collapsed the left atrium. On reintervention, a hematoma was discovered that selectively compressed the left atrium (LA).
CT and MR imaging are 2 very accurate techniques for diagnosing and quantifying pericardial effusion,20,21 although they should not be used routinely for diagnosing pericardial effusion, except in selected cases. Similarly, these 2 techniques allow study of the distribution of the effusion and, therefore, can be especially useful for patients who are poor candidates for echocardiography and can be useful for identifying localized effusions which can be visualized particularly well postcardiac surgery (although in these patients, who are generally intubated and on multiple medications, it is difficult to perform the these tests), or in some patients with pericardial tumors, such as is shown in Figure 6. It has been reported that, compared with echocardiography, MR allows better identification of small localized effusions around the vertex of the left ventricle;21 however, this finding is clinically irrelevant in most patients. In addition, MR can provide information about the nature of the pericardial effusion, and has the capacity to differentiate between low-density transudate/exudates or a hemopericardium.22 An additional advantage of using CT and MRI is that they can provide important data about the possible etiology of the pericardial effusion, especially in cases of tumor masses or adenopathy. For this reason, the use of such imaging is recommended, not for diagnosing the presence of pericardial effusion or tamponade (which should be diagnosed by other methods) but as a tool of etiological determination when neoplastic endocarditis is suspected, which must be considered in particular in patients who present with a clinical picture consistent with cardiac tamponade and who have no apparent inflammatory symptoms (pericardial pain and fever) (Figure 7).23 In patients in whom neoplastic endocarditis has already been diagnosed, CT or MR are also useful for evaluating the origin and extent of the neoplastic disease. In Table 3, we summarize the possible indications for CT and MR in patients with suspected pericardial effusion or tamponade.
Fig. 6. Magnetic resonance (MR) of a patient who presented with a clinical picture consistent with system venous hypertension with deformity of the right cardiac border on chest radiograph. A high-density mass was noted (arrows) adjacent to the right cardiac border, compromising and displacing the heart toward the left. A biopsy was performed, and it was shown to be a pericardial mesothelioma, which had been diagnosed on transthoracic echocardiogram.
Fig. 7. Chest computerized tomography (CT) of a patient who was admitted with a picture consistent with cardiac tamponade. On the CT scan (performed after pericardiocentesis) the presence of an already-identified pericardial effusion (arrow) was revealed, but allowed identification of the presence of lung tumors, adenopathy, and an image suggestive of carcinomatous lymphangitis. The final diagnosis was adenocarcinoma of the lung with metastatic neoplastic pericarditis.
CONSTRICTIVE PERICARDITIS
The etiological spectrum of constrictive pericarditis has changed recently, at least in developed countries, manifest in a decrease in tubercular etiology and an increase in the number of cases with constriction secondary to radiotherapy and cardiac surgery.24 Similarly, the manifestations of the disease have changed. In a study published in 1959,25 pericardial calcification was documented in 90% of cases of constrictive pericarditis, while in a recent series26 calcification was documented in only 27% of cases. On the other hand, the recognition of and the capacity to diagnosis constrictive pericarditis has probably improved due to a high level of suspicion for the disease, and the use of echo-Doppler and modern imaging techniques, which have allowed the identification of more unusual (and, often, less serious) forms of constriction. The result is that, although some years ago restrictive pericarditis was diagnosed only in its more severe forms (basically according to the presence of calcification on chest radiography), we have currently learned to better identify noncalcified constrictive pericarditis and other forms of constriction, such as effusive-constrictive pericarditis,27 elastic constrictive pericarditis,28 occult constrictive pericarditis,29 and transient constrictive pericarditis.30
The diagnosis of constrictive pericarditis should be suspected in all patients who present with a clinical picture consistent with right cardiac insufficiency of unknown origin, especially if, in addition, physical examination yields confirmatory results (diastolic collapse of the venous jugular pulse, protodiastolic sounds). In such cases, the documentation of pericardial calcification (Figure 8) is sufficient to establish the diagnosis and, probably, additional tests would not be necessary. When there is no pericardial calcification, the diagnosis should be based on verifying the presence of a constrictive physiology (usually by means of an echo-Doppler study) and by establishing the presence of pericardial thickening on imaging studies. The signs that suggest constriction on echo-Doppler imaging are shown in Table 4.
Fig. 8. Chest radiograph of the profile indicating extensive pericardial calcification in a patient with clinical findings typical of constrictive pericarditis. In this case it was not necessary to use additional imaging techniques.
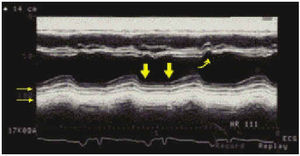
The echocardiographic finding that is most consistent with constrictive pericarditis is abnormal movement of the interventricular septum (Figure 9), which manifests as an anterior displacement that occurs during the protodiastolic rapid depletion phase and that coincides with the «y» collapse in the jugular venous pulse.31 This sign can be seen in more than 90% of cases of constrictive pericarditis. In patients who are in sinus rhythm, displacement of the interventricular septum toward the left ventricle immediately after the P-wave can also be seen on electrocardiogram. These abnormal movements of the interventricular septum are due to the changes in the transeptal pressure gradients associated with atrial contraction and with rapid protodiastolic ventricular filling. Nevertheless, none of these signs is specific to constrictive endocarditis because they can also occur in patients with a volume overload of the left ventricle, in interatrial communication, in pulmonary stenosis, in chronic obstructive pulmonary disease, and in 20% of cases of restrictive myocardiopathy, which is the disease that can cause the most difficulties in terms of differential diagnosis and constrictive pericarditis. The remaining echocardiographic signs may be helpful only in some cases. The analysis of flow using echo-Doppler provides valuable information about the type of hemodynamic change since Doppler can document the physiology of disturbances in diastolic ventricular filling. Nevertheless, in spite of the fact that an attempt has been made to establish criteria to differentiate constrictive pericarditis from restrictive myocardiopathy32,33 (the exhaustive review of which was the aim of our study), in some cases (especially in noninfiltrative restrictive myocardiopathy), it may be difficult to make a differential diagnosis with absolute certainty. For all of these reasons, the diagnosis of constrictive pericarditis must be supported by imaging technique results that show thickening of the pericardium. Transthoracic echocardiogram is not very useful to this end because of its technical limitations and the fact pericardial thickening is not homogeneous; the technique is able to show pericardial thickening in only 30% to 60% of patients (Figure 9).34,35 One study36 showed that transesophageal echocardiogram has a 95% sensitivity and an 86% specificity for detecting of pericardial thickening ≥3 mm; nevertheless, this technique is not generally used for this purpose. CT and MR are, probably, the most useful techniques for identifying and quantifying pericardial thickening, given that, on one hand, the pericardial membrane, especially the anterior portion, can be observed in practically 100% of patients and, on the other hand, these techniques have been shown to have good anatomical correlation for determining the thickness of the pericardium.37 Nevertheless, MR does not allow differentiation of fibrosis from calcification; therefore, to obtain this valuable anatomical data, it is preferable to perform a CT without contrast (Figure 10).38 The normal pericardium is a fine membrane between 1 and 3 mm thick, although its thickness is not uniform and in some areas (the caudal portion) it can measure up to 3 or 4 mm in thickness.39 Patients with constrictive pericarditis consistently have pericardiums that are more than 4 mm in thickness; however, this is not always the case, as in some patients the pericardium can have a normal appearance, at least in some areas (Figure 10). In 1 study of a series of patients with proven constrictive pericarditis, the pericardium was reported as normal on conventional CT in 21% of patients.40 This number is probably inflated; although the evaluations were performed meticulously and with an adequate suspicion for constrictive pericarditis, the researchers emphasized the fact that an apparently normal pericardium on CT or MR imaging does not permit unilaterally discarding the possible presence of constrictive pericarditis. The explanation for this phenomenon is related to the limitations of the imaging techniques themselves, since constrictive pericarditis occurs in pericardiums that are not very thickened, or can be caused primarily by visceral pericardium (constrictive epicarditis). On the other hand, the finding of pericardial thickening on CT or MR imaging is not synonymous with constrictive pericarditis. Pericardial thickening is only 1 anatomical finding (not infrequently, in inflammatory pericarditis of any etiology) that may or may not be accompanied by constriction. Therefore, the indication to perform these tests to differentiate constrictive pericarditis from restrictive myocardiopathy should be to reserve testing for patients with clinical results and external signs consistent with a constriction/restriction physiology. The possible use of CT or MR imaging to establish the differential diagnosis between constrictive pericarditis and restrictive myocarditis can be seen in Figure 11. On rare occasions, neoplasia of developing or metastatic pericardiums can cause a picture consistent with constriction rather than with tamponade. In these case, CT or MR imaging are adequate for showing nodular images of nodular tumors or other metastases, findings that are relevant for establishing the diagnosis.
Fig. 9. M-mode echocardiogram of a patient with constrictive pericarditis. Apart from the characteristic septal notch (curved arrow), in this case thickening (small horizontal arrows) and crushing (thick arrows) can be seen of the posterior pericardial movement.
Fig. 10. Chest CT of a patient with clinical constrictive pericarditis and with no calcification on chest radiograph. Areas of pericardial calcification are evident (horizontal arrows), but in other areas (anterior face, vertical arrow) the pericardium had a normal appearance. The presence of constrictive pericarditis was confirmed during the intervention.
Fig. 11. Flowchart showing the treatment of patients with a constrictive/restrictive syndrome diagnosed by means of clinical findings and external signs. Restrictive infiltrative myocardiopathy (amyloidosis, hemochromatosis) tend to be diagnosed fairly easily by analyzing clinical, analytical, and echocardiographic data. Establishing the differential diagnosis between constrictive pericarditis and idiopathic restrictive myocardiopathy is more difficult in some cases, and performing of an exploratory thoracotomy may be considered in some cases medical treatment may be considered. AIP indicates acute idiopathic pericarditis; TbP, tuberculous pericarditis; PP, purulent pericarditis; MR, magnetic resonance; CT, computed tomography.
Effusive-constrictive pericarditis is an entity in which both pericardial effusion and thickening of the visceral pericardium exist. It usually involves subacute evolutionary pericarditis that can be of diverse etiologies (idiopathic, post irradiation, tuberculous, uremic). Patients present with a clinical picture and external signs consistent with tamponade (abundant pericardial effusion, collapses, characteristic flow anomalies), and with coexistent findings suggestive of constriction (anomalous septal movement, protodiastolic sounds, «y» collapse marked by the jugular venous flow). Nevertheless, the definitive diagnosis can only be made with persistent signs of right cardiac insufficiency with findings typical of constriction after performing successful pericardiocentesis (ideally, with controlled intrapericardial pressure). Effusive-constrictive pericarditis tends to be an evolutionary disease, and patients usually wind up developing constrictive pericarditis.
In general, constrictive pericarditis is considered to be an irreversible disease, which is certainly the case in patients with chronic constrictive carditis, but there are forms of constriction that can be reversed. In the resolution phase of acute effusive acute pericarditis (idiopathic or viral), the appearance of signs suggesting constriction can be detected externally (sometimes even occurring with signs of right cardiac insufficiency) and later resolve completely (transitory cardiac constriction). The importance in recognizing this fact can promote avoidance of performing unnecessary pericardiectomy. Therefore, in the setting of signs of constriction (usually not serious) during the resolution of acute idiopathic or viral pericarditis, a wait-and-see approach should be adopted, as it is very probable that the signs will remit spontaneously. In some cases, thickening of the pericardium observed on CT later resolved. In other types of pericarditis of other etiologies (tuberculous, purulent), the possibility that the constriction may be transitory is less likely, although we have also seen resolution in some cases. Therefore, and as is shown in Figure 11, the possibility that constrictive pericarditis may be transitory must be kept in mind in cases of subacute constrictions that occur during the resolution phase of acute inflammatory pericarditis.
The term occult constrictive pericarditis was introduced by Bush in 1977 to describe a form of constrictive pericarditis, not very serious, in which only hemodynamic disturbances manifest after rapid intravenous perfusion of liquids (1000 mL of physiological serum in 6-8 minutes). Clinically, patients have symptoms that are not very severe and are nonspecific, and that apparently disappear after undergoing pericardiectomy. In our experience, on one hand, it is unusual to see patients with this clinical profile; on the other hand, the use of liquid overload to reveal a possible constriction is not a very standard procedure and its interpretation is questionable. We have actually never performed a pericardiectomy under such circumstances.
The indication for pericardiectomy is clear in patients with known chronic constrictive pericarditis and clinical evidence of cardiac insufficiency. In these cases, intervention should not be delayed, as in patients with advanced disease (chronic, NYHA functional class IV) the risk of intervention is high (surgical mortality rate: 30%-40% vs 6%-19%) and the benefit is less.24,41 The prognosis is particularly unfavorable in patients with constrictive pericarditis secondary to chest irradiation. Following surgery, the clinical improvement and normalization of hemodynamic disturbance can take weeks or months, although recovery is quicker in cases that have not progressed and in patients with noncalcified pericarditis, in which effective pericardiectomy could not be performed. In patients in NYHA functional class I with no signs of congestive cardiac insufficiency, pericardiectomy is probably not indicated, especially if, in addition, there are surgical risk factors.
Section sponsored by Laboratorio Dr. Esteve
Correspondence: Dr. J. Sagristá Sauleda.
Servei de Cardiologia. Hospital General Universitari Vall d'Hebron.
P. Vall d'Hebron, 119-129. 08035 Barcelona. España.
E-mail: jsagrist@cs.vhebron.es