.
IntroductionBased on the current European guidelines,1,2 stratification for thromboembolic risk in atrial fibrillation (AF) is largely based on clinical risk scoring systems, such as the CHADS2 score or, more recently, the CHA2DS2-VASc score.3 The net clinical benefit favors anticoagulation for almost all AF patients with the exception of those at very low risk of ischemic stroke, with a CHA2DS2-VASc score=0.4 When the CHADS2 risk score is applied, approximately 70%-80% of all patients with AF are would be eligible for oral anticoagulation (OAC), but if the CHA2DS2-VASc score is applied instead, the percentage of patients with an indication for OAC could increase to almost 94%.5
It is important to appreciate that coronary artery disease is present in around 20%-30% of patients with AF.6 Notably, nearly 10% of patients with acute coronary syndrome (ACS) have AF.7 Patients with AF presenting with an ACS or with chronic ischemic heart disease undergoing percutaneous coronary intervention (PCI) with stent implantation represent a complex management problem.8–10 Importantly, these patients have a bad prognosis.11
Current guidelines for ACS and PCI recommend the use of dual antiplatelet therapy after an ACS for 12 months irrespective of PCI, as well as after an elective stent implantation (usually 4 weeks for a bare-metal stent [BMS] and up to 12 months for a drug-eluting stent [DES]).12,13 Combined acetylsalicylic acid-clopidogrel therapy is less effective in preventing stroke than OAC alone, and OAC alone is insufficient to prevent stent thrombosis.
The recent Consensus Document by the European Society of Cardiology Working Group on Thrombosis, endorsed by the European Heart Rhythm Association and the European Association of Percutaneous Cardiovascular Intervention, suggests the use of triple therapy (TT) in patients with AF presenting with ACS or chronic ischemic heart disease undergoing stenting, including OAC plus acetylsalicylic acid 100mg per day and clopidogrel 75mg per day in the short term, followed by longer therapy with OAC plus a single antiplatelet drug; after 1 year, OAC alone with warfarin seems to be sufficient.8 Many similarities are evident with a more recent North American consensus document.9
Even in patients with a high bleeding risk (HAS-BLED risk score>3), OAC improves prognosis (reducing mortality and major adverse cardiovascular events), but with a significant increase in major bleeding.14 The combination of OAC and a single antiplatelet agent (either acetylsalicylic acid or clopidogrel) does not appear to be an optimal combination in terms of stent thrombosis, thromboembolism, and bleeding occurrence and therefore should not be prescribed as the initial management strategy following PCI with stenting.15 However, the use of TT is associated with a much higher bleeding rate.16,17 Moreover, there are other factors that also increase the rate of bleeding. These factors frequently coexist in this type of patient, such as the use of a glycoprotein IIb/IIIa inhibitor, left main or 3-vessel disease, older age (>75 years), female gender, chronic kidney disease, and a high international normalized ratio value.
In patients with AF and a moderate-high risk of stroke, with a requirement for long-term OAC, there is a need to balance stroke prevention and the risk of stent thrombosis after PCI-stenting vs the harm of bleeding with the combination antithrombotic therapy. Nonetheless, stroke risk is closely related to bleeding risk in AF patients.18,19 Thus, the Consensus Document recommends a series of strategies directed at reducing the bleeding risk in these patients.8
Use of a Radial ApproachA radial approach is the recommended elective access in this type of patient for several reasons. The femoral approach is an independent predictor of access site complications in warfarin-treated patients (a hazard ratio of 9.9).20 Furthermore, this approach allows the PCI procedure to be performed in patients treated with warfarin without complete cessation of OAC, thus avoiding bridging therapy with heparin.
Low Adjusted International Normalized RatioThe dose intensity must be carefully regulated with a target international normalized ratio of 2.0-2.5. Indeed, a study by Rossini et al.21 demonstrated a bleeding rate in patients with TT similar to that in patients receiving double antiplatelet therapy only. Unfortunately, this objective is often difficult to achieve, especially at discharge, when the maximum benefit can be provided, but also when there may be more interactions with distinct drugs.
No Heparin BridgingUntil recently, interventional cardiologists were reluctant to perform coronary catheterization in fully anticoagulated patients. The radial approach and daily problems presented by patients with AF with anticoagulant therapies changes have led to more frequent use of PCI in continuously anticoagulated patients without bridging with heparin, given the increased risk of thromboembolism and bleeding with bridging therapy. The radial approach allows the operator not to stop the OAC in unstable patients or at least to perform PCI only after a short interruption of OAC with an international normalized ratio close to the lower end of the therapeutic range. This strategy may reduce periprocedural bleeding and thromboembolic events during bridging therapy.
The duration of the TT is mainly determined by 2 variables: the clinical setting (nonmodifiable variable) and the type of stent (modifiable variable). Since the benefit of double antiplatelet therapy in patients with ACS is mainly obtained during the first few months, the type of stent may be the variable that influences the duration of TT.
Type of stent and antithrombotic therapyThere is unanimity that the use of DES should be avoided or strictly limited to clinical and anatomical situations such as long lesions, small vessels and diabetic patients, in which a significant benefit is expected as compared to BMS. The advantage with BMS is the use of TT for only 4 weeks following PCI. The benefit of DES is the reduction of target vessel revascularization but many problems arise from its use in patients with AF, including the inherent bleeding risk of the longer duration of antiplatelet therapy22; in addition, TT has been associated with major bleeding rates as high as 7%/year.23 The interruption of dual antiplatelet therapy could also result in a higher rate of stent thrombosis,24 although a hypothetical shorter duration of dual antiplatelet therapy could be recommended.25 Finally, the temporary discontinuation of anticoagulation is associated with a higher risk of thromboembolism.26
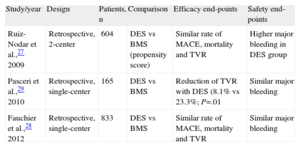
Only limited data have been provided by nonrandomized studies or retrospective registry series (Table). After a propensity score analysis, we have previously observed a similar rate of serious events and all-cause mortality during follow-up after DES compared with BMS in this population.27 Even though DES were used in lesions with a higher restenosis risk, the predictive factors for major adverse cardiac events and mortality were age, chronic renal failure, chronic AF and nonuse of coumarin at discharge. In our series, the major bleeding rate was particularly higher in the DES group, showing a clear relationship with the prolonged dual therapy or TT demanded by DES.27
Design and Most Important Findings in Published Studies Reporting on Patients With Atrial Fibrillation and Use of Drug Eluting Stents.
| Study/year | Design | Patients, n | Comparison | Efficacy end-points | Safety end-points |
| Ruiz-Nodar et al.,27 2009 | Retrospective, 2-center | 604 | DES vs BMS (propensity score) | Similar rate of MACE, mortality and TVR | Higher major bleeding in DES group |
| Pasceri et al.,29 2010 | Retrospective, single-center | 165 | DES vs BMS | Reduction of TVR with DES (8.1% vs 23.3%; P=.01 | Similar major bleeding |
| Fauchier et al.,28 2012 | Retrospective, single-center | 833 | DES vs BMS | Similar rate of MACE, mortality and TVR | Similar major bleeding |
BMS, bare-metal stent; DES, drug-eluting stent; MACE, major adverse cardiovascular events; TVR, target vessel revascularization.
Another registry analyzed the influence of the use of DES in patients with AF.28 Among 833 consecutive patients, DES was only used in 19%, with a similar ratio of serious events at follow-up after DES compared to BMS (that is, similar survival curves with a similar rate of target lesion revascularization: 2.7% vs 1.3% year of exposure; P=.15), although the use of DES was more frequent in diabetic patients and longer lesions. Notably, implantation of DES was not significantly associated with a higher risk of major bleeding. In contrast, Pasceri et al.29 reported a small series of 165 patients with a large difference in the incidence of target vessel revascularization (8.1% in DES vs 23.3% IN BMS; P=.01) during a 12-month follow-up with no differences in major bleeding.
Real life patients are usually much more complex. These AF patients are usually elderly with a high prevalence of diabetes and unfavorable coronary anatomy (multivessel disease; long, calcified small vessel lesions). Ideally, single focal lesions would be found in large vessels, where only a conventional stent would be implanted. As every interventional cardiologist knows, patients with AF have an unfavorable profile and the theoretical (guideline) recommendation of the “nonuse of DES” is not always easy to apply. In these situations, disagreement may arise among clinical cardiologists who know that the use of DES will demand an extension of TT use and hence increase the bleeding risk.
Both clinical and interventional cardiologists are aware of the guideline recommendations, and both have clear arguments for their strategies, i.e. that “DES should be avoided” and “DES should be limited to clinical and anatomical situations with high risk of restenosis”. These 2 contrasting positions must be discussed in each individual case between the clinical and interventional cardiologists, balancing the pros and cons of both strategies. A variable that should always be quantified in this discussion is the objective risk of bleeding (as assessed by the HAS-BLED score).30 Although the use of BMS should be prioritized in patients with a high restenotic risk, multiple vessel disease, etc., revascularization with DES can be an option, but always with newer second- or third-generation DES (which require shorter dual antiplatelet therapy use) and the duration of the TT should be adapted to the bleeding risk. This therapy can be prolonged for 6 or 12 months in patients with high thrombotic risk and low bleeding risk, or alternatively, reduced to 3 months in those with a high bleeding risk.
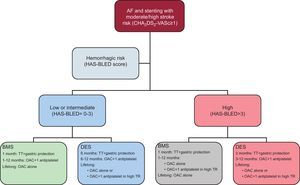
The management algorithm that we propose (Figure) is based on the recommendations of the Consensus Document by the European Society of Cardiology Working Group on Thrombosis, but gives substantial weight to the hemorrhagic risk. The use of BMS should be recommended in patients with high hemorrhagic risk. Nevertheless, DES should have their place, even in these high bleeding risk patients. In particular patients, the use of TT for the first 6 months followed by OAC + 1 antiplatelet drug for the first year can be recommended. The duration of antiplatelet therapy must take into account the thrombotic risk (e.g. “high risk” ACS; several DES; treatment of proximal left anterior descending or left main coronary artery; long lesions, etc.).
Recommendations for the duration of triple therapy and antithrombotic strategies in patients with atrial fibrillation at moderate-to-high thromboembolic risk and coronary stenting. AF, atrial fibrillation; BMS, bare-metal stent; DES, drug-eluting stent; OAC, oral anticoagulation; TR, thrombotic risk; TT, triple therapy (oral anticoagulation+acetylsalicylic acid [100mg/day]+clopidogrel [75mg/day]).
The most controversial aspect is the use of DES in patients with high hemorrhagic risk. This option is not contemplated in the Consensus Document but must be discussed because patients with a higher hemorrhagic risk are also those with a higher thrombotic and/or restenotic risk. In our series, DES were used in 47% of patients with a HAS-BLED score of≥314 and in the series by Fauchier et al.,28 in 44%. Overall, the use of BMS should be prioritized but in lesions with a high restenotic rate there should be the possibility of the use of second- or third-generation DES. Overall, the optimal antithrombotic medication should be TT for 3 months followed by OAC+1 antiplatelet drug during the first year. In patients treated with DES, with high and low hemorrhagic risk, the more chronic treatment strategy is determined by the hemorrhagic and thrombotic risk, with chronic use of OAC being used for most patients and the option of OAC+1 antiplatelet drug being reserved for only those patients with high thrombotic risk and a manageable hemorrhagic risk.
Other options may also be considered, but these will be based more on experience than on evidence-based data. Such options include a conservative management strategy for patients with high comorbidity and high bleeding risk, when there is a low or moderate ischemic risk, essentially in patients with myocardial infarction secondary to ischemia due to either increased oxygen demand or decreased supply (type 2 myocardial infarction).31 This type of presentation is common in this population due to hemodynamic compromise from the rapid AF itself, anemia or even coronary embolism. Invasive strategies would demand a change in the regimen of antithrombotic drug (i.e. longer duration of dual antiplatelet therapy), which is always associated with a higher incidence of bleeding. After individualizing each case, a conservative strategy may be evaluated in certain patients. Other options are a less aggressive revascularization procedure, treating only the culprit artery and avoiding complete revascularization, which would require more stents with a subsequent increased risk of thrombosis and restenosis. Finally, a coronary artery bypass graft should be considered in some patients to avoid a prolonged antiplatelet regimen.
Ongoing trialsMost current recommendations are based on limited evidence obtained from small, single-center and retrospectively analyzed cohorts. The debate clearly continues and will do so for a long time.
At present there are 3 randomized trials, in addition to a number of ongoing registries. The Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation (ISAR-TRIPLE) trial32 is studying the hypothesis that reducing the duration of clopidogrel therapy from 6 months to 6 weeks after DES implantation is associated with improved clinical outcomes in patients on acetyl salicylic acid and OAC. The What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing (WOEST) study33 will assess the hypothesis that the combination warfarin plus clopidogrel is superior to TT with respect to bleeding complications while equally safe with respect to the prevention of thrombotic complications in patients with AF treated with stents. The third trial is a Spanish trial, Anticoagulation in Stent Intervention Trial (MUSICA-2),34 which will try to randomize 304 patients with CHADS2<2 (low to moderate stroke risk) to dual or triple antiplatelet therapy. The primary endpoint will be a composite endpoint that includes death, myocardial infarction, stroke, embolization or stent thrombosis at 12 months.
These trials should help to define some of the potential treatment options for these patients, but they will never provide definitive solutions. The complexity of these patients, the hemorrhagic risk and high comorbidities will hamper the inclusion of patients with AF treated with stents in trials attempting to generalize a strategy.
An important issue is the availability of novel anticoagulants that will increasingly be used in place of warfarin in AF patients.35 One such novel agent, apixaban, had its Phase 3 trial (Apixaban for Prevention of Acute Ischemic Events 2 [APPRAISE-2]) in ACS stopped due to lack of benefit and potential harm by adding dual antiplatelet therapy to apixaban 5mg bid, which is the dose used for stroke prevention.36 In contrast, a low dose (2.5mg) bid regimen of rivaroxaban reduced mortality in the Anti Xa Therapy to Lower cardiovascular events in addition to standard therapy in subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction 51 (ATLAS-2) trial, but a vexing question will remain on how to manage an AF patient on the stroke prevention dose of rivaroxaban 20mg OD, who presents with an ACS or with ischemic coronary heart disease undergoing PCI with stent implantation,37 especially in the era of potent new antiplatelet agents such as prasugrel and ticagrelor.38,39
ConclusionsStent-treated patients with AF are a population at high risk and with high comorbidity. In recent years, a number of strategies supported by expert consensus documents have aimed to reduce hemorrhagic risk without increasing thrombotic risk. The use of BMS should be the cornerstone of percutaneous treatment in this population, allowing the use of TT, a more effective combination in this population but associated with increased bleeding risk, to be shortened. The use of DES should be reserved for patients with very high restenotic risk and low-moderate hemorrhagic risk, in whom the use of TT is possible. In contrast, the use of DES should be limited in patients with high bleeding risk, being reserved for only those patients or lesions with unacceptable risk of restenosis with BMS. In these cases, the use of second- or third-generation DES is recommended, and thus TT should be limited to the first 3 months.
Conflicts of interestDr. Ruiz-Nodar has received research grants and speaker's fees from Medtronic, Boston Scientific and Astra-Zeneca. Dr. Marín has served as a consultant for Bayer, BMS/Pfizer and Boehringer Ingelheim and has been on the speaker bureau for Boehringer Ingelheim, Astra-Zeneca and Boston Scientific. Dr Lip has served as a consultant for Bayer, Astellas, Merck, Sanofi, BMS/Pfizer, Daiichi-Sankyo, Biotronik, Portola and Boehringer Ingelheim and has been on the speaker's bureau for Bayer, BMS/Pfizer, Boehringer Ingelheim, and Sanofi Aventis.

![Recommendations for the duration of triple therapy and antithrombotic strategies in patients with atrial fibrillation at moderate-to-high thromboembolic risk and coronary stenting. AF, atrial fibrillation; BMS, bare-metal stent; DES, drug-eluting stent; OAC, oral anticoagulation; TR, thrombotic risk; TT, triple therapy (oral anticoagulation+acetylsalicylic acid [100mg/day]+clopidogrel [75mg/day]). Recommendations for the duration of triple therapy and antithrombotic strategies in patients with atrial fibrillation at moderate-to-high thromboembolic risk and coronary stenting. AF, atrial fibrillation; BMS, bare-metal stent; DES, drug-eluting stent; OAC, oral anticoagulation; TR, thrombotic risk; TT, triple therapy (oral anticoagulation+acetylsalicylic acid [100mg/day]+clopidogrel [75mg/day]).](https://static.elsevier.es/multimedia/18855857/0000006600000001/v1_201307091032/S1885585712003118/v1_201307091032/en/main.assets/thumbnail/gr1.jpeg?xkr=eyJpdiI6ImxISUM5b3phaWdzaUJrbHpkTFNrZ0E9PSIsInZhbHVlIjoiR3ZEb0ZWQktFUDlGRzdVb3FUMU5yZTUrVjZ4Rkt1Q0lNVTdDZmVzSnczMD0iLCJtYWMiOiJmMDIxN2U0Mjc1MmQwZTliNmIwYTNjNjdhMGM4ZTUyNTgzNGU2MzNmMjhkNzhmNWM1MjYxNGEwODc5NjBlM2YyIiwidGFnIjoiIn0=)