Over the last decade, telemedicine applied to pacemaker monitoring has undergone extraordinary growth. It is not known if telemonitoring is more or less efficient than conventional monitoring. The aim of this study was to carry out a systematic review analyzing the available evidence on resource use and health outcomes in both follow-up modalities.
MethodsWe searched 11 databases and included studies published up until November 2014. The inclusion criteria were: a) experimental or observational design; b) studies based on complete economic evaluations; c) patients with pacemakers, and d) telemonitoring compared with conventional hospital monitoring.
ResultsSeven studies met the inclusion criteria, providing information on 2852 patients, with a mean age of 81 years. The main indication for device implantation was atrioventricular block. With telemonitoring, cardiovascular events were detected and treated 2 months earlier than with conventional monitoring, thus reducing length of hospital stay by 34% and reducing routine and emergency hospital visits as well. There were no significant intergroup differences in perceived quality of life or number of adverse events. The cost of telemonitoring was 60% lower than that of conventional hospital monitoring.
ConclusionsCompared with conventional monitoring, cardiovascular events were detected earlier and the number or hospitalizations and hospital visits was reduced with pacemaker telemonitoring. In addition, the costs associated with follow-up were lower with telemonitoring.
Keywords
Cardiovascular disease is one of the leading causes of morbidity and mortality and is responsible for 30% of worldwide mortality, according to the World Health Organization.1 The incidence of cardiovascular disease has been affected by the increase in life expectancy and consequent aging population, and some of these patients require cardiovascular implantable electronic devices. The number of cardiovascular implantable electronic, devices—which includes pacemakers, implantable cardioverter-defibrillators, cardiac resynchronization therapy, and Holters—has increased at an exponential rate since the first implant2 in 1958, and continues to do so. A pacemaker is an electronic device designed to produce electrical impulses to stimulate the heart when normal physiological stimulation fails.3 The increase in the number of pacemakers implanted in the last decade, amongst other reasons, has led to the saturation of cardiology clinics.4,5
Telemonitoring (TM) consists of using electronic equipment to observe and record physiological processes while patients carry out their activities of daily living. This means the remote measurement of physiological processes such as vital signs (for example heart rate, respiratory rate, and blood pressure) and other measurements (such as blood counts, blood biochemistry, and renal production) with digital and analogue technology.6 This technology originated in the 1970s, when transtelephonic monitoring was introduced,7 and the first pacemaker that could be remotely monitored using telephone lines, cable networks, and/or broadband was introduced at the beginning of the 21st century.8 Transtelephonic monitoring was able to supply basic information, such as warning of imminent battery depletion, but it did not report problems with device functioning or the control of incorrectly programmed parameters. The introduction of telemonitoring has allowed access to a large amount of information, with the advantage that health professionals can consult it at any time. The development and expansion of pacemaker TM means that studies are required to show its efficiency compared with hospital monitoring (HM). Therefore, the aim of this study was to conduct a systematic review of economic evaluations to analyze the evidence available on resource use and health outcomes for both follow-up modalities.
METHODSSearch StrategyThe literature search was conducted on 1 December 2014, with no restrictions on language or year of publication. The databases used were MEDLINE (via PubMed), EMBASE, DARE, HTA, NHS Economic Evaluation Database (NHS EED), LILACS, IMA, CUIDEN, and the doctoral theses available on Teseo, TDR, and Dialnet. We also searched the gray literature: acts of congress, books, and academic publications; and we hand-searched bibliographic references that were considered to be of interest and that were included in systematic reviews and meta-analyses. The Boolean operators used were AND and OR. The following English search terms were used: pacemaker, telemedicine, remote consultation, home monitoring, and cost-benefit analysis. The following Spanish descriptors/key words were used: marcapasos, telemedicina, consulta remota, monitorización domiciliaria, and análisis de coste-beneficio. The key words or terms search was performed on all the selected databases in the review and on the complete article, including the title, summary, text, and key words. The inclusion criteria for studies were: a) experimental or observational design; b) studies based on complete economic evaluations, that is, studies comparing health outcomes and costs, with no exclusions for analysis method (cost-effectiveness, cost-utility, cost-benefit, and cost-minimization); c) patients with pacemakers, and d) TM compared with HM.
Data ExtractionIn December 2014, using the search strategy, 2 investigators (Antonio López-Villegas and Irene Villegas-Tripiana) independently extracted the data and read the titles and summaries of all the initially selected studies (Table 1). As stated in the study aims, articles that could potentially meet the inclusion criteria were preselected. The following month, the same 2 investigators read the full texts of the previously screened articles. When there was no consensus on the inclusion or exclusion of an article, a third investigator (Daniel Catalán-Matamoros) mediated. The variables included in the data analysis were: a) study characteristics (author, year of publication, country, study duration, sample size, age, sex, main indication for implantation, and pacemaker used), and b) analysis and main results of the variables (analysis performed, primary endpoints, secondary endpoints, health outcomes and cost outcomes). Two revisers (Carlos Martín-Saborido and Emilio Robles-Musso) independently evaluated the methodological quality of the selected articles using the checklist of López-Bastida et al9 as an assessment tool.
Search Strategy Used in MEDLINE (via PubMed)
| Search terms | |
| #1 | Search (pacemaker) OR pacemakers |
| #2 | Search telemedicine |
| #3 | Search remote consultation |
| #4 | Search home monitoring |
| #5 | Search (((cost-benefit analysis) OR cost benefit analysis) OR cost benefit) OR cost-benefit |
| #6 | #1 and #2 or #3 or #4 |
| #7 | #1 and #5 |
| #8 | #6 and #7 |
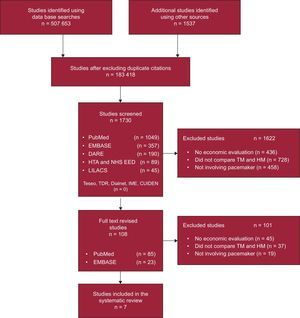
The literature search identified 1730 articles. After the full texts of 108 potentially relevant studies had been revised, 7 articles10–16 met the selection criteria (Figure) and were included in the subsequent synthesis of evidence. The references from the 101 excluded articles are available in the supplementary material.
The review included 3 experimental studies and 4 descriptive/observational studies, and aimed to evaluate the results on quality of life, effectiveness, safety, reliability, and costs of TM of pacemakers compared with HM.10–16
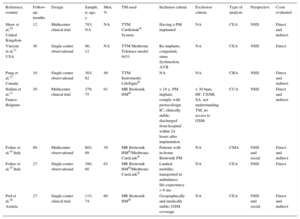
The main characteristics of the studies are summarized in Table 2. The selected studies represent a total of 2876 enrolled patients, 1303 of them in randomized clinical trials. The sample sizes of the studies varied (96-802 patients). The mean age of the patients in 5 of the studies12–16 was 81.40±6.77 years (95% confidence interval, 73.00-89.80 years). The main indication for pacemaker implantation was atrioventricular block.12–16 The study period ranged from 10 months to 80 months. All of the selected studies used the same pacemaker model in both follow-up arms, with the exception of the studies by Folino et al,14,15 which used 2 different pacemaker models in the HM group. None of the selected studies stated if monitoring systems were previously being used for all pacemakers followed up by the hospital.
Characteristics of the Selected Studies
| Reference, country | Follow-up, months | Design | Sample, n; age, y | Men, % | TM used | Inclusion criteria | Exclusion criteria | Type of analysis | Perspective | Costs evaluated |
|---|---|---|---|---|---|---|---|---|---|---|
| Shaw et al,10 United Kingdom | 12 | Multicenter clinical trial | 783; NA | NA | TTM Cardiotrak® System | Having a PM implanted | NA | CEA | NHS | Direct and indirect |
| Vincent et al,11 USA | 36 | Single-center observational | 96; 12 | NA | TTM Medtronic Teletrace model 9431 | Re-implants, congenital, sinus dysfunction, AVB | NA | CEA | NHS | Direct |
| Pang et al,12 Canada | 10 | Single-center observational | 303; 82 | 49 | TTM Instromedix LifeSigns® | NA | NA | CBA | NHS | Direct and indirect |
| Halimi et al,13 France-Belgium | 20 | Multicenter clinical trial | 379; 75 | 61 | MR Biotronik HM® | > 18 y, PM implant, comply with protocol/sign IC; clinically stable; discharged from hospital within 24 hours after implantation | < 30 bpm, HF, CS/MI, SA, not understanding TM, no access to GSM | CUA | NHS | Direct and indirect |
| Folino et al,14 Italy | 80 | Multicenter observational | 802; 88 | 39 | MR Biotronik HM®/Medtronic CareLink® | Patients with in-home Biotronik PM | NA | CMA | NHS and social | Direct and indirect |
| Folino et al,15 Italy | 27 | Single-center observational | 398; 88 | 63 | MR Biotronik HM®/Medtronic CareLink® | Limited mobility; transported in ambulance; life-expectancy > 6 mo | NA | CEA | NHS | Direct |
| Perl et al,16 Austria | 27 | Single-center clinical trial | 115; 74 | 60 | MR Biotronik HM® | Geographically and medically stable; GSM coverage | NA | CEA | NHS and social | Direct and indirect |
AVB, atrioventricular block; bpm, beats per minute; CBA, cost-benefit analysis; CEA, cost-effectiveness analysis; CMA, cost-minimization analysis; CS, cardiac surgery; CUA, cost-utility analysis; HF, heart failure; GSM, global system for mobile communications; HM®, Home Monitoring®; IC, informed consent; MI, myocardial infarction; NA, not available; NHS, national health system; PM, pacemaker; SA, systemic anticoagulation; TM, telemonitoring; TTM, transtelephonic monitoring.
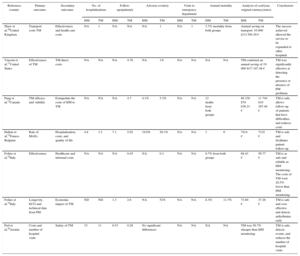
Table 3 contains the primary and secondary endpoints analyzed in each of the studies, as well as the most significant results. A cost-effectiveness analysis was performed in 4 of the publications.10,11,15,16 The number of pacemaker replacements ranged from 7 to 123,14,15 and device longevity ranged from 6.7 years to 8.3 years.12–15 Only the study by Halimi et al13 specified the mean hospital stay, which was 34% shorter in the TM group. That was also the only study that evaluated quality of life with the SF-36 questionnaire, finding no significant differences between the 2 groups.
Analysis and Main Results of the Variables Evaluated
| Reference, country | Primary outcomes | Secondary outcomes | No. of hospitalizations | Follow-ups/patient/y | Adverse events/y | Visits to emergency department | Annual mortality | Analysis of cost/year, original currency/euros | Conclusions | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HM | TM | HM | TM | HM | TM | HM | TM | HM | TM | HM | TM | ||||
| Shaw et al,10United Kingdom | Transport costs TM | Effectiveness and health care costs | NA | 1 | NA | NA | NA | 1 | NA | 1 | 3.7% mortality from both groups | Annual saving on transport: 10 000 £/13 594.30 € | The success achieved allowed the service to be expanded to other hospitals | ||
| Vincent et al,11United States | Effectiveness of TM | TM direct costs | NA | NA | NA | 4.76 | NA | 1% | NA | NA | NA | NA | TM conferred an annual saving of 19 000 $/17 247.48 € | TM was significantly effective at detecting the presence or absence of PM problems | |
| Pang et al,12Canada | TM efficacy and viability | Extrapolate the costs of HM to TM | NA | NA | NA | 4.7 | 4.1% | 5.3% | NA | NA | 12 deaths from both groups | 88 230 $78 038.21 € | 11 744 $10 387.40 € | TM is safe, allows follow-up of patients that have difficulties, and reduces costs | |
| Halimi et al,13France-Belgium | Rate of MAEs | Hospitalization, costs, and quality of life | 4.8 | 3.2 | 7.1 | 5.92 | 19.0% | 20.1% | NA | NA | 1 | 0 | 7414 € | 7125 € | TM is safe and facilitates patient follow-up |
| Folino et al,14Italy | Effectiveness | Healthcare and informal costs | NA | NA | NA | 0.45 | NA | 0.3 | NA | NA | 8.7% from both groups | 68.43 € | 56.77 € | TM is as safe and reliable as HM monitoring. The costs of TM were 20.5% lower than HM monitoring | |
| Folino et al,14Italy | Longevity, ECG and technical data from PM | Economic impact of TM | ND | ND | 1.3 | 2.6 | NA | 52% | NA | NA | 8.3% | 11.7% | 73.80 € | 37.26 € | TM is safe and cost-effective and detects arrhythmias early |
| Perl et al,16Austria | Costs and number of hospital visits | Safety of TM | 15 | 11 | 0.53 | 0.29 | No significant differences | NA | NA | NA | NA | TM was 58.7% cheaper than HM monitoring | TM is safe, detects events, and reduces the number of hospital visits. | ||
ECG, electrocardiogram; HM, hospital monitoring; MAE, major adverse event; NA, not available; PM, pacemaker; TM, telemonitoring.
The study by Pang et al12 showed that, of all the emergency transmissions in the TM group, only 8% were detections of significant arrhythmic events; this number rose to 52% in the study by Folino et al.15 In TM groups, 0.6% to 1.9% of patients attended hospital for pacemaker reprogramming.14,15 In the OEDIPE trial,13 20.1% of patients in the telemonitoring group had an adverse event vs 19.0% in the hospital group. Annual mortality12–15 was between 0% and 11.7%.
Cost AnalysisNone of the selected studies reported the costs of implementing TM. Vincent et al11 reported that the costs of TM were paid by the hospital, whereas Folino et al14,15 reported that the costs were paid by the pacemaker manufacturers. To facilitate the comparison between the different currencies included in the selected publications, the cumulative annual inflation was calculated from the year following publication of the article to December 2014; a direct conversion was then made from each currency into euros according to the exchange rate at 20 February 2015. The 7 studies included in the review reported that TM costs were lower than HM follow-up costs (Table 3). In the WEST-SCOTLAND study,10 the potential savings for the National Health Service associated with ambulance transport were estimated to be 13 594 euros annually, by replacing one follow-up system with the other. This estimation was for 637 patients pertaining to 3 hospitals in Ayrshire, from a total of 783 patients included in the WEST-SCOTLAND study.10 The study by Vincent et al11 showed that there would have been a saving of 17 247 euros over the 3 years that the research lasted, if the 96 patients included in the study had had the data transmission system instead of emergency department visits. The differences in economic impact are evident in a number of studies12,13 that found TM to be up to 4 to 8 times cheaper than the conventional option. Patients in the TM group had a mean of 2 visits less (TM vs HM, 5.92 vs 7.1), according to the OEDIPE trial.13 Folino et al14 estimated a mean difference of 20.5% in costs between the 2 groups. Furthermore, in the same study, if patients had to travel to hospital by ambulance, the difference rose to 66.5%. Other studies report separate results according to type of cost per year; for example in SAVE-HM,16 staffing costs were significantly lower in the TM group (18.0±41.3 euros vs 22.4±26.9 euros; P<.003). Also, informal costs associated with the transport used by patients to attend hospital were included, and it was shown that for kilometers travelled, the absolute costs of the TM group were lower than those of the standard outpatient group by almost 60% (872.14 euros vs 2162.78 euros). Table 4 contains the costs associated with each monitoring method.
Costs Included in Each Follow-up Modality
| Reference, country | Telemonitoring | Hospital monitoring |
|---|---|---|
| Shaw et al,10 United Kingdom | StaffTelephoneTransport | StaffTelephoneTransport |
| Vincent et al,11 United States | Monthly cost of routine and emergency visits (including PM analysis)Emergency department costs excluding PM analysis | Monthly cost of routine and emergency visits (included PM analysis)Emergency department costs excluding PM analysis |
| Pang et al,12 Canada | Staff (nurses)Hospital visitsEquipment rentalTelephone calls | Staff (physician + nurse)Hospital servicesAllowancesTransport costs |
| Halimi et al,13 France-Belgium | StaffLaboratoryIndirect costs (physicians and paramedics)Transport | StaffLaboratoryIndirect costs (physicians and paramedics)Transport |
| Folino et al,14 Italy | Health care costs (physician, nurse, and transport)Informal costs (transport and productivity)NHS (PM check costs) | Health care costs (physician, nurse, and transport)Informal costs (transport and productivity)NHS (PM check costs) |
| Folino et al,15 Italy | NHS (visit costs)Staff (physician + nurse) | NHS (visit costs)Staff (physician + nurse)Transport |
| Perl et al,16 Austria | StaffTransport | StaffTransport |
NHS, National Health System; PM, pacemaker.
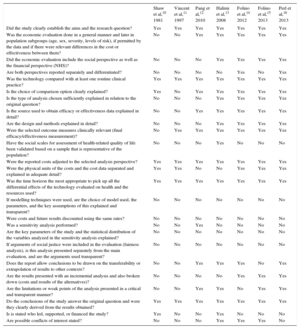
The variables evaluated were scored on yes/no answers regarding the presence or absence of the criterion analyzed (Table 5). If on final review of the article, a parameter was not found, the response “no” was put in the table, meaning that the study did not include that parameter.
Checklist for Methodological Quality of the Studies
| Shaw et al,10 1981 | Vincent et al,11 1997 | Pang et al,12 2010 | Halimi et al,13 2008 | Folino et al,14 2012 | Folino et al,15 2013 | Perl et al,16 2013 | |
|---|---|---|---|---|---|---|---|
| Did the study clearly establish the aims and the research question? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the economic evaluation done in a general manner and later in population subgroups (age, sex, severity, levels of risk), if permitted by the data and if there were relevant differences in the cost or effectiveness between them? | No | No | Yes | Yes | Yes | Yes | Yes |
| Did the economic evaluation include the social perspective as well as the financial perspective (NHS)? | No | No | No | Yes | Yes | Yes | Yes |
| Are both perspectives reported separately and differentiated? | No | No | No | No | Yes | No | Yes |
| Was the technology compared with at least one routine clinical practice? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the choice of comparison option clearly explained? | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Is the type of analysis chosen sufficiently explained in relation to the original question? | No | No | No | Yes | Yes | Yes | Yes |
| Is the source used to obtain efficacy or effectiveness data explained in detail? | No | No | Yes | Yes | Yes | Yes | Yes |
| Are the design and methods explained in detail? | No | No | No | Yes | Yes | Yes | Yes |
| Were the selected outcome measures clinically relevant (final efficacy/effectiveness measurement)? | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Have the social scales for assessment of health-related quality of life been validated based on a sample that is representative of the population? | No | No | No | Yes | No | No | No |
| Were the reported costs adjusted to the selected analysis perspective? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the physical units of the costs and the cost data separated and explained in adequate detail? | Yes | Yes | No | No | Yes | Yes | Yes |
| Was the time horizon the most appropriate to pick up all the differential effects of the technology evaluated on health and the resources used? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| If modelling techniques were used, are the choice of model used, the parameters, and the key assumptions of this explained and transparent? | No | No | No | No | No | No | No |
| Were costs and future results discounted using the same rates? | No | No | No | No | No | No | No |
| Was a sensitivity analysis performed? | No | No | Yes | No | No | No | No |
| Are the key parameters of the study and the statistical distribution of the variables analyzed in the sensitivity analysis explained? | No | No | No | No | No | No | No |
| If arguments of social justice were included in the evaluation (fairness analysis), is this analysis presented separately from the main evaluation, and are the arguments used transparent? | No | No | No | No | No | No | No |
| Does the report allow conclusions to be drawn on the transferability or extrapolation of results to other contexts? | No | No | Yes | Yes | Yes | No | Yes |
| Are the results presented with an incremental analysis and also broken down (costs and results of the alternatives)? | No | No | No | No | Yes | Yes | Yes |
| Are the limitations or weak points of the analysis presented in a critical and transparent manner? | No | No | Yes | Yes | No | Yes | Yes |
| Do the conclusions of the study answer the original question and were they clearly derived from the results obtained? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is is stated who led, supported, or financed the study? | Yes | No | No | Yes | No | No | No |
| Are possible conflicts of interest stated? | No | No | No | Yes | Yes | Yes | No |
NHS, National Health System; No, absence of criterion; Yes, presence of criterion.
Two studies14,16 had a higher overall score for methodological quality, with 17 out of a possible 25 points. The lowest scoring study was by Shaw et al,10 which scored 7 points. The publications evaluated had a mean of 12.71±4.72 points (95% confidence interval, 8.35-17.08). The main findings were as follows:
- •
Two articles14,16 included health and social perspectives.
- •
Five studies12–16 clearly stated the source of the effectiveness data, and 4 of them stated the design and methods used to obtain the data.13–16
- •
None of the studies applied modelling techniques or discounts for costs and benefits, or conducted a sensitivity analysis.
- •
In 4 publications12–14,16 conclusions could be drawn on the extrapolation of the results to other contexts.
- •
Three articles14–16 reported the results for costs and effects on health of the 2 methods separately. However, 3 studies did not present study limitations in a critical and transparent way.10,11,14
- •
In all the publications,10–16 the conclusions answered the original research questions.
- •
None of the selected studies included cost-effectiveness or cost-benefit ratios.
The findings of this review reveal that TM of pacemakers, compared with HM, did not lead to significant differences in health-related quality of life or number of cardiovascular events. With TM, such events were detected and treated earlier, which reduced the number of hospitalizations and routine and emergency hospital visits. In addition, the costs of follow-up were lower than those of HM. The variable economic impact of this technology among the countries and regions where the studies were conducted was greatly influenced by transport, healthcare staff, work absences, and informal care.
Effectiveness and Clinical Safety of TelemonitoringThe studies included in the review showed that TM reduced the number of hospital follow-up visits.13,14,16 The results were similar to those published in the COMPAS trial,17 which showed a reduction of 55% in the number of hospital visits for patients with pacemakers.
The development and expansion of pacemaker TM have allowed its safety and reliability to be proven.10–16 The early detection of cardiovascular events and/or abnormal device functioning allowed patients to receive early treatment, meaning a significant reduction in the number and seriousness of hospitalizations.18–20 In a study conducted over 12 months, with 897 patients with pacemakers, Crossley et al21 showed that such events were detected 2 months earlier in the TM group than in the HM group (5.7 months vs 7.7 months). In 2 earlier studies,18,19 conducted over 15 months and 12 months, respectively, and involving patients with automatic cardioverter-defibrillators and cardiac resynchronization therapy, the response time for such events was 22 days to 36 days with HM, but were reduced to 2.0 days to 4.6 days in the home monitoring group. The ECOST20 and EVATEL22 trials showed that there were no significant differences between the 2 follow-up methods in the number of adverse events detected, which was consistent with the results obtained in 3 publications of this review.13,15,16
Quality of life was analyzed only in the trial by Halimi et al,13 showing no significant differences between the 2 follow-up arms. The results were consistent with those of the COMPAS17 and ECOST20 trials, which used the SF-36 questionnaire, and with a recently published trial23 that used the EQ-5D questionnaire.
Evaluation of the methodological quality of the selected studies showed that there was wide heterogeneity among them, with more recent studies obtaining higher scores. The results presented in this review show the difficulty in using current criteria to evaluate the methodological quality of studies conducted decades ago.10,11 However, certain parameters were consistent in all the selected studies, such as pre-established aims and research questions, comparison of both modalities of follow-up, adjustment of costs accumulated to the selected analysis perspective, and adaptation of the time horizon to the aims of the study. Likewise, none of the studies applied modelling techniques, discounted costs, performed sensitivity analyses, explained key parameters or statistical distribution of the variables, performed fairness analyses, or included the cost-effectiveness and cost-benefit ratios.
Economic Aspects of TelemonitoringThe follow-up of patients with cardiovascular disease not only involves a significant burden for the patients, but also requires a significant amount of economic resources from the various publicly-funded national health systems.
The results reported in the OEDIPE trial13 showed that TM reduced hospital stays by 50%. However, the total costs were not significantly different from those of hospital follow-up, due to the reimbursement system based on diagnosis-related groups.
The information supplied in this review confirms that, due to the reduction in the number of hospital visits and hospital admissions, this technology displays a clear potential to reduce health care costs associated with staffing (health professionals and administrative staff) as well as other aspects related to follow-up (such as transport costs and maintenance of clinics). These results are similar to those of other economic evaluations on implantable cardioverter-defibrillators.19,24,25 Raatikainen et al24 showed that by reducing the number of hospital visits, the cost per patient was reduced by 41% (524 euros). This saving increased to 60.9% in the study by Elsner et al,26 who estimated an annual saving of 712.31 euros per patient due to a 63.2% decrease in the number of visits and associated transport costs. In a subsequent trial by Crossley et al,18 patient hospital stays were reduced by 18% with TM, representing a saving of 1659 dollars per patient year. Fauchier et al27 estimated an economic saving of 2149 euros per patient with TM over the 5-year useful life of implantable cardioverter-defibrillators.
Limitations and Future Research LinesOur analysis has several limitations. The first is the low number of selected studies (n=7) and participants included (n=2852), which was mainly because TM technology is used less frequently than HM. The second limitation is the methodological heterogeneity of the selected studies, given that they did not use modelling techniques or discount costs and outcomes, nor did they explain the key study parameters or the statistical distribution of the variable analyzed in the sensitivity analysis. The third limitation is the lack of any clinical trials measuring mid- and long-term outcomes, fundamentally due to TM being a relatively new technology. The fourth limitation is the 32-year difference between the selected studies; over this time, technology has changed enormously, and in this review the similarities and differences can be seen between the 2 technologies, which have been used in different spatiotemporal settings. Finally, the cost-effectiveness studies were less generalizable than the effectiveness studies, as they were dependent on both study duration and context. Even so, their significance is enormous, as they facilitate decision-making for the various professionals involved. This is the first systematic review of economic evaluations to analyze the health outcomes and resource use associated with pacemaker TM. By describing the results obtained in both follow-up arms, this review has allowed current knowledge on the subject to be updated, thus providing tools for future decision-making and new health policies.
In future research, economic evaluations would be advisable, comparing both monitoring modalities, and including cost-effectiveness ratios and the informal costs associated with follow-up. The time horizon should be mid- to long-term.
This review can be used by both healthcare managers and cardiology service professionals to promote the sustainability of the current healthcare systems.
CONCLUSIONSPacemaker TM detects cardiovascular events earlier reduces hospitalizations, the number of hospital visits, and the associated costs of follow-up.
FUNDINGThis project was partly funded by the European Economic Area Grant 008/ABELCM/2014A of the NILS Science and Sustainability Program 2014 call for research.
CONFLICTS OF INTERESTNone declared.