To analyze the rate of patients admitted for acute coronary syndrome who concomitantly received acetylsalicylic acid, statins, and angiotensin-converting enzyme inhibitors at discharge, and to analyze interhospital variability in the prescription of these drugs and its potential prognostic impact.
MethodsInterhospital variability in drug prescription was estimated using the intraclass correlation coefficient and median odds ratio (hierarchical analysis). Cox regression analysis was used to estimate the risk of death or myocardial infarction associated with prescription of all 3 agents at 2-years of follow-up.
ResultsIn total, 489 (53.3%) of 917 patients were prescribed all 3 agents. The rate was similar in patients with hypertension and diabetes (56.8%). There was significant variability among centers in the prescription of the 3 drugs at discharge (from 23% to 77% of patients). Hypertension (odds ratio=1.93; 95% confidence interval, 1.42-2.61), ejection fraction < 45% (odds ratio=2.2; 95% confidence interval, 1.44-3.37), being in a clinical trial (odds ratio=1.89; 95% confidence interval, 1.24-2.88), and renal failure (odds ratio=0.53; 95% confidence interval, 0.29-0.94) were associated with prescription of the 3 drugs. After adjustment for these factors, residual variability persisted (intraclass correlation coefficient 0.046 [95% credibility interval, 0.007 to 0.192]; median odds ratio=1.46 [95% credibility interval, 1.16-2.32]). There was no clear association between the prescription of all 3 drugs and the risk of events during follow-up (hazard ratio=0.81, 95% confidence interval, 0.55-1.18; P=.27).
ConclusionsThe prescription rate for acetylsalicylic acid, angiotensin-converting enzyme inhibitors, and statins after acute coronary syndrome is suboptimal, varies among centers, and is possibly related to different health care approaches.
Keywords
Improved survival rates have been observed in patients discharged after an acute coronary syndrome (ACS) with guideline-indicated medications, such as antiplatelet agents, statins, angiotensin-converting enzyme (ACE) inhibitors, and beta-blockers.1–4 Adherence to these medications has also been associated with improved prognosis.5
However, the prescription rate of guideline-indicated medications for patients with ischemic heart disease is far from optimal, and it has been suggested that the underestimation of patient risk and poor adherence to guidelines are potential factors underlying suboptimal prescribing.6 It has also been suggested that the situation is similar in other contexts, such as heart failure.7 The use of fixed doses of several drugs in a single pill (known as a polypill) for patients with chronic conditions such as ischemic heart disease could increase patient adherence and adherence to guidelines by physicians.8,9 Polypills have recently been added to the therapeutic arsenal in Spain.
The ACDC study (Adherence to antiplatelet treatment in acute Coronary synDrome patients after Catheterization) was a prospective registry of patients with at least 1 drug-eluting stent admitted to 29 Spanish hospitals.10–12 This study used the ACDC database to analyze the proportion of patients admitted with ACS who were prescribed 3 drugs (acetylsalicylic acid, statins, and ACE inhibitors), interhospital variability in the prescription rate of the 3 drugs, and the characteristics of the patients and hospitals associated with this variability. This study also analyzed if there was an association between being discharged with prescriptions for all 3 drugs and the composite event rate of cardiovascular death, ACS, or stroke at 2-years of follow-up.
METHODSThe ACDC study has already been published.10–12 The study was a prospective multicenter cohort study, which included 29 hospitals. Almost all the public and private hospitals in Catalonia that performed percutaneous procedures were included. It was not logistically possible to include all other Spanish hospitals and thus a representative sample of 12 hospitals from the autonomous communities was selected. The inclusion criterion was a patient with a drug-eluting stent, thus ensuring the comprehensive consecutive inclusion of patients without exclusion criteria. Patients were included in the study from 28th January, 2008 to 28th April, 2008. The study collected information on type of hospital, demographic variables, risk factors, patient history, reason for admission, procedure performed, complications during hospitalization, and treatment at discharge. Most of the variables had standard definitions, which were reviewed with the fieldworkers during the preparatory meetings.
Seven hospitals were excluded from the analysis because they had less than 14 patients; multilevel statistical analysis requires a minimum number of patients per cluster for the estimates to be considered robust.13
Statistical AnalysisOf the 1965 patients who were discharged during the ACDC study, 968 patients (49%) had been admitted for ACS; of these patients, 917 (47%) patients were discharged from the 22 hospitals included in the present analysis.
Quantitative variables are expressed as mean and standard deviation or as median and interquartile range. Discrete variables are expressed as proportions. Two groups of patients were analyzed: Those who had been discharged with prescriptions for all 3 drugs, and those who had been discharged with prescriptions for 1 or 2 drugs. Differences between groups were evaluated using the Student t test or the Mann-Whitney U test (according to the data distribution), and the chi-square test.
Variability AnalysisThe main aim of the analysis was to evaluate interhospital variability in the prescription rate for all 3 drugs at discharge and to analyze if any variability was due to the patients or hospitals having different characteristics, such as the volume of patients treated and the type of funding (public or private). A 3-step multilevel logistic regression model was used for this analysis. Firstly, an empty model was constructed in which the random constant term measured interhospital variability in the ratio of patients treated with 3 drugs. Secondly, several individual patient characteristics were included to analyze the extent to which interhospital variations in prescriptions could be attributed to differences in the patients treated at each hospital. All the baseline characteristics that differed between groups were included (P < .2). Age and sex were included as fixed adjustment variables. Once adjusted for patient characteristics, interhospital variability should be zero if variability in the rate of patients prescribed all 3 drugs depended exclusively on interhospital variability in the number of treated patients. Finally, a third model included hospital characteristics: the number of patients with stents implanted during 1 year, whether the hospital was private or public, and whether it was a university hospital or not. Odds ratios (OR) were estimated as measures of association. The multilevel logistic regression models were estimated by assuming independent covariance using the procedure included in the R statistical software package, version 3.2.0.
The change in variability among hospitals was measured at each step by calculating the percentage change in interhospital variance between the more complex model and the simpler model. The intraclass correlation coefficient (ICC) and median OR (MOR) were estimated to measure the size of interhospital variance. The ICC can be interpreted as the proportion of total variance in the variable considered that could be attributed to interhospital variation. The MOR was defined as the median value of the estimated OR in a “high-risk” hospital vs a “low-risk” hospital after repeatedly and randomly selecting 2 hospitals. The MOR was used to express the association between an individual's likelihood of being discharged with a prescription for all 3 drugs and the hospital discharging the patient. A MOR of 1 indicated that there was no interhospital variation in the prescription rate; however, if the MOR strongly differed from 1, then some characteristic of the hospital was affecting an individual's likelihood of being discharged with a prescription for all 3 drugs (ie, some interhospital variation remained unexplained). Bayesian estimation was used to obtain 95% credibility intervals (95%CrI) for the ICC and MOR.
Model calibration and discrimination were estimated using the Hosmer-Lemeshow test and the receiver operating characteristic curve. In both cases, the hierarchical structure of the data was taken into account when predicting the likelihood of a patient being discharged with prescriptions for all 3 drugs.
The methods and formulas used for the multilevel analysis are presented in Merlo et al.14
Survival AnalysisCox regression models were used to analyze if discharge with a prescription for all 3 drugs was associated with a higher composite event rate of “ACS, cardiovascular death, or stroke” at 2 years of follow-up. Firstly, a model was constructed that included the main predictors of the composite event. Candidate variables were those that were associated with the event in the binary analysis (P<.2; see supplementary material). Backward and forward stepwise modeling was used to select the best model and the explanatory variable “3 drugs at discharge” was subsequently included in the model.
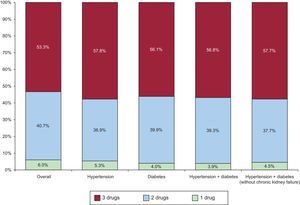
RESULTSOf the 917 patients with ACS discharged from the 22 hospitals during the study period, 55 patients (6%) were prescribed antiplatelet drugs alone, 373 (40.7%) were prescribed 2 drugs, and 489 (53.3%) all 3 drugs. Of the patients prescribed 2 drugs, 100% received antiplatelet therapy, 11.2% of whom received an ACE inhibitor, and 88.8% a statin. When various subgroups of patients were considered depending on the presence of hypertension and diabetes or the absence of kidney failure, defined as a baseline creatinine concentration of less than 1.4mg/dL (first analysis at admission), there was no significant change in the proportion of prescriptions for all 3 drugs (Figure 1).
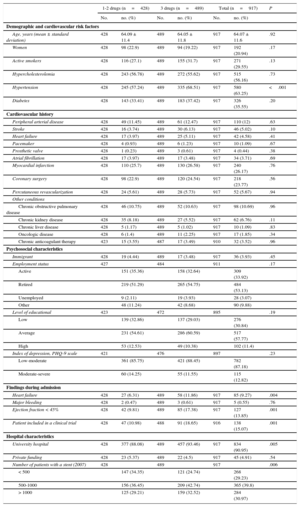
Table 1 shows the differences in baseline characteristics between patients prescribed 1 or 2 drugs at discharge vs patients prescribed all 3 drugs. The former group of patients tended to have higher rates of chronic kidney failure, whereas the latter group had higher levels of hypertension. There were no significant differences between patients in socio-cultural characteristics, including their level of depression, which was assessed with the PHQ (Patient Health Questionnaire). Heart failure during admission, ejection fraction < 45%, and being included in a clinical trial were more frequent in patients prescribed all 3 drugs at discharge. Being discharged from a university hospital and a higher hospital activity index was also more frequent among patients discharged with all 3 drugs.
Baseline Characteristics According to the Number of Drugs Prescribed at Discharge
| 1-2 drugs (n=428) | 3 drugs (n=489) | Total (n=917) | P | ||||
|---|---|---|---|---|---|---|---|
| No. | no. (%) | No. | no. (%) | No. | no. (%) | ||
| Demographic and cardiovascular risk factors | |||||||
| Age, years (mean ± standard deviation) | 428 | 64.09 ± 11.4 | 489 | 64.05 ± 11.8 | 917 | 64.07 ± 11.6 | .92 |
| Women | 428 | 98 (22.9) | 489 | 94 (19.22) | 917 | 192 (20.94) | .17 |
| Active smokers | 428 | 116 (27.1) | 489 | 155 (31.7) | 917 | 271 (29.55) | .13 |
| Hypercholesterolemia | 428 | 243 (56.78) | 489 | 272 (55.62) | 917 | 515 (56.16) | .73 |
| Hypertension | 428 | 245 (57.24) | 489 | 335 (68.51) | 917 | 580 (63.25) | <.001 |
| Diabetes | 428 | 143 (33.41) | 489 | 183 (37.42) | 917 | 326 (35.55) | .20 |
| Cardiovascular history | |||||||
| Peripheral arterial disease | 428 | 49 (11.45) | 489 | 61 (12.47) | 917 | 110 (12) | .63 |
| Stroke | 428 | 16 (3.74) | 489 | 30 (6.13) | 917 | 46 (5.02) | .10 |
| Heart failure | 428 | 17 (3.97) | 489 | 25 (5.11) | 917 | 42 (4.58) | .41 |
| Pacemaker | 428 | 4 (0.93) | 489 | 6 (1.23) | 917 | 10 (1.09) | .67 |
| Prosthetic valve | 428 | 1 (0.23) | 489 | 3 (0.61) | 917 | 4 (0.44) | .38 |
| Atrial fibrillation | 428 | 17 (3.97) | 489 | 17 (3.48) | 917 | 34 (3.71) | .69 |
| Myocardial infarction | 428 | 110 (25.7) | 489 | 130 (26.58) | 917 | 240 (26.17) | .76 |
| Coronary surgery | 428 | 98 (22.9) | 489 | 120 (24.54) | 917 | 218 (23.77) | .56 |
| Percutaneous revascularization | 428 | 24 (5.61) | 489 | 28 (5.73) | 917 | 52 (5.67) | .94 |
| Other conditions | |||||||
| Chronic obstructive pulmonary disease | 428 | 46 (10.75) | 489 | 52 (10.63) | 917 | 98 (10.69) | .96 |
| Chronic kidney disease | 428 | 35 (8.18) | 489 | 27 (5.52) | 917 | 62 (6.76) | .11 |
| Chronic liver disease | 428 | 5 (1.17) | 489 | 5 (1.02) | 917 | 10 (1.09) | .83 |
| Oncologic disease | 428 | 6 (1.4) | 489 | 11 (2.25) | 917 | 17 (1.85) | .34 |
| Chronic anticoagulant therapy | 423 | 15 (3.55) | 487 | 17 (3.49) | 910 | 32 (3.52) | .96 |
| Psychosocial characteristics | |||||||
| Immigrant | 428 | 19 (4.44) | 489 | 17 (3.48) | 917 | 36 (3.93) | .45 |
| Employment status | 427 | 484 | 911 | .17 | |||
| Active | 151 (35.36) | 158 (32.64) | 309 (33.92) | ||||
| Retired | 219 (51.29) | 265 (54.75) | 484 (53.13) | ||||
| Unemployed | 9 (2.11) | 19 (3.93) | 28 (3.07) | ||||
| Other | 48 (11.24) | 42 (8.68) | 90 (9.88) | ||||
| Level of educational | 423 | 472 | 895 | .19 | |||
| Low | 139 (32.86) | 137 (29.03) | 276 (30.84) | ||||
| Average | 231 (54.61) | 286 (60.59) | 517 (57.77) | ||||
| High | 53 (12.53) | 49 (10.38) | 102 (11.4) | ||||
| Index of depression, PHQ-9 scale | 421 | 476 | 897 | .23 | |||
| Low-moderate | 361 (85.75) | 421 (88.45) | 782 (87.18) | ||||
| Moderate-severe | 60 (14.25) | 55 (11.55) | 115 (12.82) | ||||
| Findings during admission | |||||||
| Heart failure | 428 | 27 (6.31) | 489 | 58 (11.86) | 917 | 85 (9.27) | .004 |
| Major bleeding | 428 | 2 (0.47) | 489 | 3 (0.61) | 917 | 5 (0.55) | .76 |
| Ejection fraction < 45% | 428 | 42 (9.81) | 489 | 85 (17.38) | 917 | 127 (13.85) | .001 |
| Patient included in a clinical trial | 428 | 47 (10.98) | 488 | 91 (18.65) | 916 | 138 (15.07) | .001 |
| Hospital characteristics | |||||||
| University hospital | 428 | 377 (88.08) | 489 | 457 (93.46) | 917 | 834 (90.95) | .005 |
| Private funding | 428 | 23 (5.37) | 489 | 22 (4.5) | 917 | 45 (4.91) | .54 |
| Number of patients with a stent (2007) | 428 | 489 | 917 | .006 | |||
| < 500 | 147 (34.35) | 121 (24.74) | 268 (29.23) | ||||
| 500-1000 | 156 (36.45) | 209 (42.74) | 365 (39.8) | ||||
| > 1000 | 125 (29.21) | 159 (32.52) | 284 (30.97) | ||||
Unless otherwise indicated, data are expressed as no. (%).
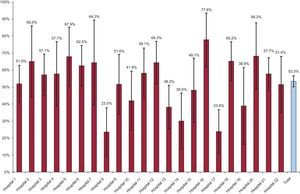
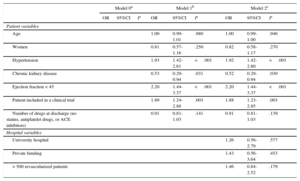
Figure 2 shows interhospital variability in the rate of patients prescribed 1, 2, or 3 drugs at discharge. In the unadjusted multilevel model, interhospital variability was 23%, with an ICC of 0.066 (95%CrI, 0.023 to 0.204) and an MOR of 1.58 (95%CrI, 1.30-2.39) (Table 2). After adjustment for age, sex, and the number of drugs other than the 3 analyzed, an increased prescription rate for all 3 agents was associated with several baseline patient characteristics, especially hypertension (OR=1.93; 95% confidence interval [95%CI], 1.42-2.61), ejection fraction < 45% (OR=2.2; 95%CI, 1.44-3.37), and inclusion in a clinical trial (OR=1.89; 95%CI, 1.24-2.88). On the other hand, chronic kidney failure was associated with a lower prescription rate for all 3 drugs (OR=0.53; 95%CI, 0.29-0.94). A model adjusted for these variables showed that interhospital variability was reduced by 8%, but residual variability remained (ICC, 0.061 [95%CrI, 0.018-0.186]; MOR=1.55 [95%CrI, 1.27-2.28]), suggesting that a factor unrelated to the patient profile is associated with different prescription rates for the 3 drugs. Finally, no association was found between the variables of being a university hospital, type of funding, and hospital activity index and an increased prescription rate for all 3 drugs. After inclusion of these variables in the model, significant variability remained among hospitals (ICC, 0.046 [95%CrI: 0.007 to 0.192]; MOR=1.46 [95%CrI, 1.16-2.32]), suggesting that the care process shows variability that remains unexplained by individual patient characteristics or by the amount of hospital care.
Factors Associated With Interhospital Variability in the Prescription of 3 Drugs
| Model 0a | Model 1b | Model 2c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| Patient variables | |||||||||
| Age | 1.00 | 0.99-1.01 | .980 | 1.00 | 0.99-1.00 | .946 | |||
| Women | 0.81 | 0.57-1.16 | .250 | 0.82 | 0.58-1.17 | .270 | |||
| Hypertension | 1.93 | 1.42-2.61 | <.001 | 1.92 | 1.42-2.60 | <.001 | |||
| Chronic kidney disease | 0.53 | 0.29-0.94 | .031 | 0.52 | 0.29-0.94 | .030 | |||
| Ejection fraction < 45 | 2.20 | 1.44-3.37 | <.001 | 2.20 | 1.44-3.37 | <.001 | |||
| Patient included in a clinical trial | 1.89 | 1.24-2.88 | .003 | 1.88 | 1.23-2.85 | .003 | |||
| Number of drugs at discharge (no statins, antiplatelet drugs, or ACE inhibitors) | 0.91 | 0.81-1.03 | .141 | 0.91 | 0.81-1.03 | .139 | |||
| Hospital variables | |||||||||
| University hospital | 1.26 | 0.56-2.79 | .577 | ||||||
| Private funding | 1.43 | 0.56-3.64 | .453 | ||||||
| > 500 revascularized patients | 1.46 | 0.84-2.52 | .179 | ||||||
95%CI, 95% confidence interval; 95% CrI, 95% credibility interval; ACEI, angiotensin-converting enzyme inhibitors; ICC, intraclass correlation coefficient; MOR, median odds ratio; OR, odds ratio.
Model 0: variance, 0.233; CCI, 0.066 (95% CrI, 0.023-0.204); ORM=1.58 (95% CrI, 1.30-2.39); calibration, 7.49 (P=.48); discrimination, 0.64 (P<.001).
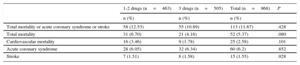
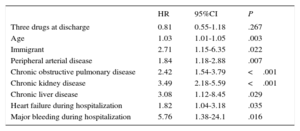
Table 3 shows that the raw event rate was slightly lower in patients prescribed all 3 drugs at 2-years of follow-up, without reaching statistical significance. A multivariate model showed that there was no clear association between being discharged with a prescription for all 3 drugs and the risk of events during follow-up (hazard ratio=0.81; 95%CI, 0.55-1.18; P=.27) (Table 4).
Raw Event Rate in Each Group After 2 Years of Follow-up
| 1-2 drugs (n=463) | 3 drugs (n=505) | Total (n=968) | P | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Total mortality or acute coronary syndrome or stroke | 58 (12.53) | 55 (10.89) | 113 (11.67) | .428 |
| Total mortality | 31 (6.70) | 21 (4.16) | 52 (5.37) | .080 |
| Cardiovascular mortality | 16 (3.46) | 9 (1.78) | 25 (2.58) | .101 |
| Acute coronary syndrome | 28 (6.05) | 32 (6.34) | 60 (6.2) | .852 |
| Stroke | 7 (1.51) | 8 (1.58) | 15 (1.55) | .928 |
Risk of Major Events Associated With Prescription of 3 Drugs at 2 Years of Follow-up
| HR | 95%CI | P | |
|---|---|---|---|
| Three drugs at discharge | 0.81 | 0.55-1.18 | .267 |
| Age | 1.03 | 1.01-1.05 | .003 |
| Immigrant | 2.71 | 1.15-6.35 | .022 |
| Peripheral arterial disease | 1.84 | 1.18-2.88 | .007 |
| Chronic obstructive pulmonary disease | 2.42 | 1.54-3.79 | <.001 |
| Chronic kidney disease | 3.49 | 2.18-5.59 | <.001 |
| Chronic liver disease | 3.08 | 1.12-8.45 | .029 |
| Heart failure during hospitalization | 1.82 | 1.04-3.18 | .035 |
| Major bleeding during hospitalization | 5.76 | 1.38-24.1 | .016 |
95%CI, 95% confidence interval; HR, hazard ratio.
This study showed that, at discharge, less than 60% of patients admitted for ACS in the ACDC study were prescribed the 3 drugs recommended by the main scientific societies. This rate remained almost unchanged when we considered the patient subgroup with a strong indication for all 3 drugs, such as those with hypertension and diabetes. In addition, there was significant interhospital variability in the prescription rate for all 3 drugs. Although interhospital variability was associated with certain characteristics of the patients, residual variability remained after adjustment for these factors. This result suggests that there are differences in the healthcare process that are not explained by the distinct characteristics of the patients, but which may be explained by differences in the healthcare practices of each hospital. Finally, although the composite event rate of cardiovascular death, ACS, and stroke was slightly lower in patients who had been discharged with a prescription for all 3 drugs, the association was inconclusive.
It has been repeatedly stated that prognosis is improved by physician adherence to guideline-based treatment instructions regarding prescribing drugs of proven efficacy.1–5 However, “suboptimal” adherence to the guidelines on ischemic heart disease and other chronic diseases is common both in Spain and Europe.15 In Spain, there has been a recent and progressive increase in the rates of prescribing antiplatelet agents, ACE inhibitors, and statins in patients discharged after an ACS. For example, the PRIAMHO II study (2000) reported an ACE inhibitors prescription rate of around 45%,16 the MASCARA study (2008) reported a rate of around 55% in a similar population,17 and the DIOCLES (2015) reported a rate of 79%.18 Although less marked, a similar situation exists in relation to statins and antiplatelet agents. In the present study, although the overall ACE inhibitors prescription rate at discharge (56.8%) was similar to that in the MASCARA study (around 55%), the prescription rate for all 3 drugs together was 53%. Although previous studies have not addressed the prescription rate for all 3 drugs at discharge, it is assumed that there has been a parallel increase in the rate of prescribing each drug.
Several studies have tried to identify the factors that explain, even partially, suboptimal drug prescribing despite the guideline recommendations. Although risk underestimation by physicians is a factor associated with suboptimal prescribing,6 there is a strong association between variability in prescribing and, in the final analysis, suboptimal prescribing, and multiple guidelines and recommendations on ACS that often overlap and even differ from each other, and the speed in which innovations or therapeutic variations are incorporated in daily practice.15
In the present study, less than 60% of patients who had been admitted with ACS were discharged with a prescription for acetylsalicylic acid, statins, and ACE inhibitors. Although contraindications or poor tolerance cannot be excluded as possible causes of the nonprescription of these drugs, this percentage barely increased in the patient subgroup with more indications, such as hypertension and diabetes, and without contraindications, such as kidney failure. Although the latter disease could deter physicians from prescribing certain drugs, particularly ACE inhibitors, it does not seem to be a determining factor. Significant interhospital variability was found in prescription rates, which ranged from just over 23% to just over 77% in patients discharged with a prescription for all 3 agents. The factors that could explain this variability include different patient characteristics by hospital and different hospital characteristics according to 3 variables: type of funding, being a university hospital, and hospital activity index.
Several patient variables, such as hypertension, low ejection fraction, and kidney failure are associated with a higher rate (the first 2 variables) or a lower rate (the third variable) of prescribing the 3 drugs. However, although expected, this association does not explain the significant interhospital variability. In fact, interhospital variability remained after adjustment for these 3 variables and variables such as age, sex, and the number of drugs prescribed at discharge that differed from the 3 drugs studied. This result suggests that specific patient characteristics alone do not explain the different rates of prescribing 3 drugs at discharge. Although the bivariate analysis showed that being treated at a university hospital and a higher hospital activity index were associated with a higher prescription rate for all 3 drugs, the association did not reach statistical significance after adjustment for individual patient variables. This result may suggest that patient characteristics differ as a function of these 2 hospital characteristics. Regardless, when both characteristics were included, there was still interhospital variability in prescribing all 3 agents. These differences in prescribing behavior deserve further study.
The aim of this study was to describe interhospital variability in prescribing and poor physician adherence to the guideline recommendations, rather than to investigate their causes or the conditions that favor them in the setting of ACS in Spain. Other studies on ACS in Spain have already described interterritorial variability and variability in access to specific tests and the use of specific drugs according to the type of hospital.19,20 Studies on interterritorial variations in the prognosis of ACS suggest that the rate of use of specific tests and recommended drugs may be an underlying explanatory factor.21 Although there may be many underlying factors, multidrug regimens have been associated with variability in prescribing and specifically with the low rate of prescribing all 3 drugs. The use of the polypill could increase the rate of prescribing drugs with a class IA recommendation in the guidelines and could be more cost-effective than conventional treatment. For these reasons, some leading researchers have appealed for their inclusion in the model list of essential medicines developed by the World Health Organization.9 If they had been included, the possible impact of using the polypill in Spain would have been shown by an increased rate of prescribing the 3 drugs and a decrease in interhospital variability.
Although there was an association between a slightly lower major event rate at 2-years of follow-up and prescription of all 3 drugs at discharge, the association did not reach statistical significance. This finding should be interpreted in the setting of the ACDC study, which included only patients with drug-eluting stents; the risk profile of these patients was more favorable than that of other patients in studies that have shown a clear association.3,22
LimitationsThe main limitation of this study is that the ACDC study was conducted in 2008 and so extrapolation of the findings to the present time should be undertaken with caution. In terms of changes in healthcare practices or the introduction of therapeutic innovations, there seems to be no compelling reason to suspect that the current situation of prescribing recommended drugs is very different to that of a few years ago. As mentioned, the ACDC study included only patients with at least 1 drug-eluting stent and excluded patients with ACS but without a drug-eluting stent; however, it is unlikely that these criteria introduced a selection bias when we estimated prescribing behavior. On the other hand, selection bias may have been introduced by using convenience sampling methods rather than random sampling methods to select the hospitals. However, any such sampling bias is more likely to have underestimated the magnitude of the findings regarding the low prescription rate for all 3 drugs and interhospital variability in prescribing, given that the participation of hospitals in the study may have led to better clinical practice than normal. The findings of this article should be interpreted in view of the fact that it only analyzed the prescription rates for the 3 drugs in patients discharged after an ACS. It could be hypothesized that, in an outpatient care setting, the prescription rate for all 3 agents might increase soon after discharge. Finally, although no statistically significant association was found between prescription of all 3 agents and the risk of major events at 2 years of follow-up, it should be taken into account that the analysis had relatively low statistical power, which was due to the use of a selected sample of patients with a drug-eluting stent, who had a lower overall risk than patients included in comprehensive registries of ACS.
FUNDINGThe ACDC study was funded with a grant from the Health Research Fund (Fondo de Investigación Sanitaria PI07/90031), an unconditional grant from Bristol-Myers-Squibb, and an unconditional grant from Laboratorios Ferrer. These organizations and institutions were not involved in the design of the study, data collection, analysis, and interpretation, or in the preparation of the manuscript.
CONFLICTS OF INTERESTNone declared.
A. Ribera-Solé, P. Cascant-Castelló, J.R. Marsal-Mora, G. Permanyer-Miralda, B. García del Blanco, G. Martí, V. Serra, I. Otaegui, B. Serra, J.F. Muñoz, O. Abdul Jawad-Altisent, S. Valdivieso, I. Roca, and D. García-Dorado (H.U. Vall d’Hebron, Barcelona); J. Mauri-Ferré, E. Nofrerías, O. Rodríguez-Leor, C. Olliete, and N. Salvatella (H.U. Germans Trias i Pujol, Badalona, Barcelona); M.C. López-Pérez (C. Sagrada Familia, Barcelona); M. Sabaté, and S. Brugaletta (H. de la Sta. Creu i Sant Pau, Barcelona); J. Casanova-Sandoval, F. Worner-Diz, L. Barta, and E. Piñol (H.U. Arnau de Vilanova, Lleida); J.A. Gómez-Hospital (H.U. de Bellvitge, L’Hospitalet Llobregat, Barcelona); B. Pujol-Iglesias, J. Bassaganyas, and M. Puigfel (H.U. Dr. Josep Trueta, Girona); M. Jiménez-Kockar, T. Martorell, X. Freixa, and S. Federico (H. Clinic, Barcelona); M.A. Cepas (C. Quirón, Barcelona); N. Batalla-Sahún, and J. Bureba-Sancho (H.U. Sagrat Cor, Barcelona); E. Blanco-Monteseirín, and E. Larrousse (C. Corachán, Barcelona); J. Guarinos, M. Bono-Más, J. Massoni, J. Mateo, A. Minguella, J. Vidal, and A. Comiñas (H.U. Joan XXIII, Tarragona); E. Martín, M.J. Fernández de Muniain-Comajuncosa, and M. Rugat-Fernández (C.C. Sant Jordi, Barcelona); A. Serra-Peñaranda, F. Miranda-Guardiola, G. Sierra, J.L. Triano, and B. Vaquerizo (H. del Mar, and C. Teknon, Barcelona); T. Ber-Muñoz, and G. Otero-Soriano (H. De Barcelona, Barcelona); D. Sanmiguel-Cervera (H.U. Dr. Peset, Valencia); R. Raso-Raso (H.U.P. La Fe, Valencia); A. Iñiguez-Romo, E.M. Sánchez-Hernández, and M.A. Martínez (H. Meixoeiro, Vigo, Pontevedra); J.R. Rumoroso-Cuevas, A. Subinas-Elorriaga, J. Onaindia, and M. Sábada (H. Galdakao, Galdakao, Bizkaia); R. Trillo-Nouche, M. Gutierrez-Feijoo, and E. González-Babarro (H.U. Santiago, A Coruña); M.L. Capote-Toledo (H.C. San Carlos, Madrid); L. Goicolea, J. Goicolea, A. Blasco, and M. Pérez-Requena (H.U. Puerta de Hierro, Majadahonda, Madrid); L. Iñigo-García, O. Sanz-Vázquez, J.F. Muñoz-Bellido, C. García-Jarillo, and M. Pombo (H. Costa del Sol, Marbella, Málaga); R. Ruiz-Salmerón, M.J. Álamo-López, A. Romero-González, J.C. Dorado, C. Márquez, J.A. Muñoz-Campos, F. Reina, S. Santos, N. García-Fernández, and M. Madueño (H.U. Virgen Macarena, Sevilla); J. Sánchez-Gila, J.A. Herrador, J.C. Fernández-Guerrero, M. Guzmán, A. Bracero, and J. Blanca-Castillo (C.H. Jaén, Jaén); M. Ruiz-Lera e I. Madrazo (H.U. Marqués de Valdecilla, Santander).