Keywords
INTRODUCTION
Renal dysfunction is a recognized independent predictor of a poor prognosis in patients with heart failure and is a common finding in this patient population. In large registries of patients hospitalized for acute decompensated heart failure (ADHF), around 30% of patients have moderate or severe renal dysfunction, and this figure can exceed 50% when mild renal dysfunction is included in the estimation.1-5
Patients hospitalized for ADHF can present worsening renal function (WRF), which can lengthen the hospital stay. In some studies, WRF is associated with increased mortality and additional hospitalizations during follow-up.6,7
The aim of this study was to determine the incidence and risk factors of WRF in patients admitted for ADHF, and the 1-year prognostic implications of presenting WRF during hospitalization.
METHODS
A retrospective, observational cohort study was carried out in 200 consecutive patients admitted to the coronary unit of Hospital Italiano de Buenos Aires with a diagnosis of ADHF from January 1, 2006 to March 31, 2007. The criteria for coronary unit admission were hypertension, requirement for continuous intravenous infusion of medication (inotropic agents, diuretics, vasodilators), or supplementary oxygen requirement. Patients were classified according to the categories established in the diagnosis and treatment guidelines of the European Society of Cardiology (type I, acute decompensated heart failure, de novo or as decompensation of chronic heart failure; type II, acute hypertensive heart failure; type III, acute pulmonary edema; type IV, cardiogenic shock; type V, high-output failure; and type VI, predominantly right heart failure).8,9 The patients' functional capacity before hospitalization was classified according to the New York Heart Association (NYHA) criteria. Patients were excluded if they presented acute ischemic syndrome, severe primary valvular disease, pulmonary thromboembolism, cardiac tamponade, or heart failure following cardiac surgery, or if they had undergone organ transplantation and were receiving immunosuppressive therapy. Patients were also excluded if they had multiorgan failure or sepsis, or if they had undergone contrast-enhanced imaging studies during hospitalization. It was decided to exclude patients receiving chronic dialysis treatment and those who required dialysis during the first 24 hours of hospitalization because they constitute a particular group with more severe renal disease.
Routine laboratory analyses, including daily serum urea and creatinine levels, were performed in all patients. The glomerular filtration rate (GFR) was estimated with the simplified MDRD (Modification of Diet in Renal Disease) formula, which is accepted as a valid method for estimating glomerular filtration in patients with heart failure.10,11
Baseline renal function at admission was considered normal12 at a GFR >90 mL/min/1.73 m2, mildly decreased at 60 to 90 mL/min/1.73 m2, moderately decreased at 60 to 30 mL/min/1.73 m2, and severely decreased at <30 mL/min/1.73 m2.
Worsening renal function was established on 2 required criteria: an increase in serum creatinine by at least 0.3 mg/dL in the absolute value and additionally, by at least 25% with respect to the baseline value.13 Left ventricular function assessed by echocardiography was considered preserved when the ejection fraction was ≥50%.
The aims of this study were to determine the following: a) the frequency of WRF and factors predictive of WRF during hospitalization; b) the frequency and factors predictive of mortality or rehospitalization for heart failure at 1 year of follow-up in groups with and without WRF; and c) the duration of hospital stay (days) according to the presence or absence of WRF.
Statistical Analysis
Assuming a 2:1 ratio of patients without WRF relative to those with WRF and with an estimated rate of events at 1 year (death or rehospitalization for heart failure) of 40% in patients with WRF, we calculated a required sample size of 200 patients to detect a 50% reduction in risk at a 95% confidence interval (CI) and power of 80% in the group without WRF compared to those with WRF.
Continuous variables are expressed as the arithmetic mean and standard deviation (SD) or as the median and interquartile range, depending on whether or not they showed a gaussian distribution. Discrete variables are expressed as percentage. Continuous data with gaussian distribution were compared with the Student t test and those with a non-gaussian distribution, with the Wilcoxon rank-sum test. Discrete data were compared with the c2 test or Fisher test. All variables that presented a P value of <.1 in the univariate analysis were included in the multivariate models. Multivariate logistic regression analysis was used to identify variables that were independent predictors of WRF, and a multivariate Cox proportional hazards analysis was used to detect variables predictive of death or rehospitalization for heart failure. Because creatinine participated in the calculation of the GFR, 2 models were tested in the multivariate analyses for WRF, death, and rehospitalization: one that included only creatinine and one that included only the GFR. The model that showed the better association with the endpoint analyzed was ultimately chosen.
The heart-failure-related mortality and rehospitalization rates were analyzed with Kaplan-Meier curves, and differences between the groups with and without WRF were compared with the log-rank test.
Statistical significance was accepted at a P value of <.05. All analyses were performed with Stata 7.0 (Stata Statistical Software, version 7.0, Stata Corporation).
RESULTS
Characteristics of the Total Population
The mean age of the 200 patients included was 78 (14) years, and 43% were women. The mean serum creatinine at admission was 1.57 (0.6) mg/dL and the GFR was 59.5 (17) mL/min/1.73 m2. Among the total of patients at admission, 18% presented normal GFR values, 27% mildly deteriorated renal function, and 55% moderately or severely deteriorated function. Other patient characteristics are shown in Table 1. The patients' clinical presentation of heart failure according to the European Society of Cardiology and their usual functional capacity prior to hospitalization are described in Table 2. According to the pre-established criteria, 46 patients (23%) experienced WRF during hospitalization.
Characteristics of Patients with Worsening Renal Function
The patient group that experienced WRF and underwent univariate analysis was 10 years older on average than the group without WRF, and included a higher percentage of persons older than 80 years of age and a larger number with an ischemic etiology. Renal function at admission, assessed by the serum creatinine value and GFR, was significantly poorer in the group with WRF, as was systolic blood pressure.
There were no differences in the other variables analyzed (Table 1). The clinical presentation according to the European Society of Cardiology guidelines was similar in patients with and without WRF, with the exception of type IV (cardiogenic shock), which was associated with WRF in all cases. Additionally, there were no differences in prehospitalization NYHA functional class between the 2 groups (Table 2).
Before hospitalization, the group who later experienced WRF was receiving significantly less intensive treatment with angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARBs) and antialdosterone agents, with no differences in the remaining medication (Table 3).
During hospitalization, all patients received intravenous furosemide, but the total dose was significantly higher in those who developed WRF. Among the total series, 23% of patients were using intravenous inotropic drugs, with a nonsignificant trend to higher use in the group with WRF; the less intensive use of ACEI or ARBs and antialdosterone agents was maintained (Table 4).
Variables that obtained a P value of <.1 in the univariate analysis and those with recognized biological importance (age, sex) were included in the multivariate analysis.
The variables considered for inclusion in the multivariate analysis were age older than 80, sex, ischemic etiology, diabetes mellitus, chronic obstructive pulmonary disease, atrial fibrillation, left ventricular ejection fraction <50%, serum creatinine concentration, GFR values, systolic blood pressure on admission <90 mm Hg, NYHA functional class >II, use of ACEI or ARBs, antialdosterone agents, intravenous furosemide dose, and use of intravenous inotropic treatment.
The independent predictors of WRF were age older than 80, renal failure at admission established as GFR <60 mL/min/1.73 m2, and systolic blood pressure on admission <90 mm Hg (Table 5).
Prognosis
The mean follow-up was 416 (143) days, and follow-up information was available for 96% of patients. The combined endpoint of death or rehospitalization for heart failure at 1 year was observed in 66 patients (33%), 22 (47.8%) in the group with WRF and 44 (28.2%) in the group without WRF. Thirty-seven patients died (18.5%), 12 (26%) in the group with WRF and 25 (16%) in the group without WRF. Excluding the 20 patients (10%) who died during the initial hospitalization (7 [5.2%] with WRF and 13 [8.3%] without WRF), and 8 patients lost to follow-up, 29 patients were rehospitalized (16.9%), 10 (29.4%) who had presented WRF and 19 who had not (13.7%).
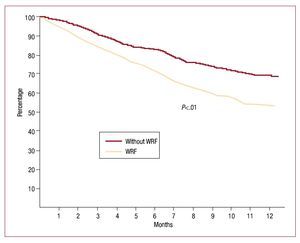
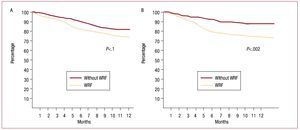
Survival free of heart failure rehospitalization, analyzed by Kaplan-Meier curves and evaluated with the log-rank test, was significantly lower (P<.01) in the group that experienced WRF (Figure 1). When the 2 outcomes were analyzed separately, survival showed no statistically significant differences (hazard ratio [HR] = 1.61; 95% CI, 0.75-2.23; P=.1), but rehospitalization was significantly more common in the WRF group (HR=2.38; 95% CI, 1.68-2.95; P=.002) (Figure 2).
Figure 1. Kaplan-Meier curve of heart failure rehospitalization-free survival by log-rank test. WRF indicates worsening renal function.
Figure 2. Kaplan-Meier curves analyzed by log-rank test. A: survival analysis. B: patients free of rehospitalization for heart failure. WRF indicates worsening renal function.
The median [interquartile range] hospitalization time was 9 [6-16] days in patients with WRF and 4 [2-8] days in those without WRF (P<.05).
In the multivariate analysis for death or rehospitalization, the same variables as those analyzed for WRF were included, and WRF was added as another variable. Variables with a P value of <.1 on univariate analysis and those with recognized biological relevance were included in the multivariate model. WRF was found to be independently associated with the combined endpoint (adjusted HR = 1.65; 95% CI, 1.12-2.67). Other variables that showed an independent association were age over 80 years, GFR <60 mL/ min/1.73 m2, and prior functional capacity >II (all variables, P<.05) (Table 6).
DISCUSSION
The social and economic importance of heart failure is undeniable. It is a prevalent condition with a high economic impact on the health system.14 Renal failure is a comorbid condition that is often associated with heart failure and adversely affects the prognosis.2-5,15-17 In patients hospitalized for ADHF, various mechanisms can lead to WRF: the patient's hemodynamic status, activation of neurohumoral mechanisms, the action of inflammatory cytokines, and use of drugs that relieve the symptoms, but can worsen renal function.18,19
Low blood pressure and decreased volume per minute, with the resulting decrease in renal flow, are basic mechanisms leading to alterations in renal function. The renal congestion observed in right heart failure also produces changes in intrarenal hemodynamics.20 The mechanisms that compensate for this situation can worsen renal function. Stimulation of the renin-angiotensin-aldosterone system and sympathetic nervous system can lead to an even greater decrease in renal flow. Sodium retention exacerbated by these compensating mechanisms and water retention due to the increase in vasopressin secretion can aggravate renal congestion.18,21,22 Cytokine concentration, which is higher in severe heart failure, can produce arterial hypertension and further worsen renal perfusion.23 In this consecutive series of patients admitted for ADHF, who in terms of age, sex distribution, ventricular function and comorbidities are a representative sample of patients admitted to cardiac intensive care units of general hospitals, 78% presented some degree of renal failure at admission, and in 55% the grade was at least moderate (GFR<60 mL/min/1.73 m2). In this vulnerable situation, it is understandable that the same mechanisms that triggered ADHF, such as tachyarrhythmia, anemia, and infection can worsen renal function, and that the drug therapy applied, such as diuretics and vasoactive agents, can lead to WRF. WRF occurred in 23% of cases, a percentage consistent with the reported rates in several studies.7,13,24-26
The definition of WRF is not uniform. Some authors establish WRF on an absolute increase in creatinine of 0.3 mg/dL,6,27 others use 0.5 mg/dL,24 and still others cite a 25% increase with respect to baseline or an increase greater than 2 mg/dL.25 One study required a 25% increase in plasma urea or a 25% drop in GFR.26 In our study, as in another recent report, a serum creatinine increase of at least 0.3 mg/dL was required in addition to a 25% increase relative to baseline. These criteria allowed correction of the differences in the GFR drop according to the baseline creatinine value.13
Predictors of Worsening Renal Function
Several predictors of WRF have been reported in the literature. Renal dysfunction before or at the time of hospitalization is cited in most studies.6,7,13,24,25 Other predictive factors include atrial fibrillation and acute pulmonary edema,6 advanced age,7 prior functional capacity and ejection fraction,13 diabetes mellitus, hyponatremia, diastolic failure,24 high diuretic requirements,13,25,27 and use of calcium channel blockers.27 In one retrospective study performed in more than 1000 patients, a history of heart failure, diabetes mellitus, creatinine >1.5 mg/ dL or systolic pressure >160 mm Hg at admission enabled creation of a score to stratify the risk of WRF: the higher the score, the higher the risk of WRF.28
In our analysis, because of the link between creatinine and calculation of GFR, these 2 variables were incorporated separately in various multivariate models. The GFR was seen to have a stronger association with WRF than creatinine concentration. Patients with moderate or severe renal dysfunction on admission (GFR<60 mL/min/1.73 m2) had a 2-fold higher risk of WRF than those with better GFR results. Although the findings of most studies are consistent with this information, in the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) study, WRF showed no association with creatinine concentration or GFR at admission. When the population was dichotomized according to GFR, those with GFR<60 mL/min/1.73 m2 showed only a nonsignificant trend to greater WRF. This difference with respect to our study may be due to the fact that patients with creatinine >3.5 mg/dL were excluded from ESCAPE and the average GFR was much higher than that of our population.29
Age was another independent predictor of WRF. Patients who experienced WRF were significantly older, and the risk of WRF was seen to increase with increasing age. The third independently associated variable was low blood pressure. This link can be explained by hemodynamic phenomena and the compensating mechanisms triggered by heart failure.
One issue currently under discussion is whether greater drug use can lead to WRF or whether patients needing more drugs are more severely ill and have a greater probability of experiencing WRF. Some studies have shown that WRF is associated with more intensive diuretic use.13 Although the patient group with WRF received more loop diuretics in our series, the association was lost after application of multivariate analysis. The WRF group received ACEI or ARBs and antialdosterone agents less often before and during hospitalization, probably because of their more severe renal deterioration and because WRF limited their use. Our data support the hypothesis that this population was older and at higher risk, ischemic etiology was more common, cardiogenic shock was observed only in this group (which received more diuretics), and there was a greater tendency to use inotropic drugs during hospitalization.
Prognostic Value of Worsening Renal Function
Patients with WRF remained hospitalized for a lengthier time. This finding is consistent with the information published to date.6,7,13,24,25,27 Although we did not analyze the higher cost resulting from prolongation of the hospital stay, the implications are obvious. It has been reported that, after adjusting for potential confounding variables, WRF is associated with longer hospitalization and higher economic cost.30
Based on the number of admissions at our hospital and sample size calculations, we opted to use a combined endpoint of death or heart failure rehospitalization at 12 months of follow-up. Apart from the limitation resulting from the number of patients and recognizing that the variable death is more important, we considered that combining death with a nonfatal event such as rehospitalization for heart failure has clinical and economic relevance, and is associated with a higher future risk of death.31
After WRF was adjusted for other clinical variables, it retained independent value for predicting death or rehospitalization for heart failure. This was because of the higher frequency of readmissions. Mortality was lower (without reaching statistical significance) in the group with WRF, but the statistical power of the study did not suffice to detect changes in mortality.
Various studies have reported an increase in mortality during hospitalization7,24,27 and up to 60 days after discharge in patients with WRF26; nevertheless, in several of them, multivariate analysis was not performed to identify the independent value of WRF.7,24,26 Few studies have continued follow-up after hospital discharge and included a multivariate analysis.13,25
Not all the published information concurs with these findings. In one multicenter European study, patients with WRF required lengthier hospitalization, but they did not present greater mortality or hospital readmissions.6 WRF was defined by an increase in serum creatinine of >0.3 mg/dL. Nonetheless, it should be noted that in the study by Metra et al,13 when the definition was based only on a serum creatinine increase >0.3 mg/ dL, WRF lost independent value to predict death or heart failure readmissions. In the ESCAPE study,29 and in the present study, baseline real function was associated with a poorer long-term prognosis (death or rehospitalization at 6 months), but the WRF did not predict the long-term events. This can be explained by the fact that the population differed: mean age 56 years (78 years in our series) and mean GFR, 71.4 mL/min/1.73 m2 (59.5 mL/min/1.73 m2 in our patients).
One interesting datum (although without statistical value) that merits further study in a larger series, was the fact that patients with an admission GFR <60 mL/min/1.73 m2 who experienced WRF had a higher risk of the combined endpoint than those with better baseline renal function (HR=2.55; 95% CI, 0.6-4.3; P=.1). This supports the concept that renal function has important prognostic implications in this population. The other variables with independent prognostic value (advanced age and poor previous functional capacity) are common findings in studies on prognostic factors in heart failure.
The retrospective nature of this study is a limitation, but this drawback is in part minimized by the fact that each patient had a single, electronic history, which allowed precise information to be obtained. Evidently, the exclusion of some unavailable variables from the multivariate analysis as well as unknown confounding variables may have modified the results. Although there are guidelines for prescribing medication in the ambulatory phase, complete treatment data were not available; hence, an influence of medication on the long-term outcome cannot be ruled out. The conclusions of this study cannot be generalized to all patients hospitalized for heart failure, but may be applicable to patients admitted to a coronary unit with characteristics similar to our study population.
CONCLUSIONS
Worsening renal failure is a common complication in patients hospitalized for ADHF and is associated with lengthier hospitalization and a higher rate of death or heart failure readmissions at 1 year, particularly readmissions. Clinical and laboratory predictors are available to enable identification at hospital admission of patients at a higher risk of experiencing WRF.
ABBREVIATIONS
ADHF: acute decompensated heart failure
CI: confidence interval
GFR: glomerular filtration rate
WRF: worsening renal function
Correspondence: Dr. C.A. Belziti.
Avda. Rivadavia 4216, Piso 3, Depto. 4. Ciudad de Buenos Aires (1205). Argentina.
E-mail: cesar.belziti@hospitalitaliano.org.ar
Received July 8, 2009.
Accepted for publication November 25, 2009.