Cardiac resynchronization therapy is associated with improved quality of life and reduced morbidity and mortality in patients with severe ventricular dysfunction and wide QRS. However, its role in the reduction of ventricular arrhythmias is more controversial.
MethodsWe compared the incidence of ventricular arrhythmias in patients who were undergoing cardiac resynchronization therapy with an implantable cardioverter-defibrillator in terms of the degree of echocardiographic response to resynchronization. Patients were classified in 3 subgroups;super-responders, responders, and nonresponders.
ResultsWe included 196 patients who were followed up for a median 30.1 months [interquartile range, 18.0-55.1 months]. We recorded the presence of ventricular arrhythmias in 37 patients (18.8%); 3 patients (5.9%) in the super-responder group had ventricular arrhythmias vs 14 (22.2%) among the responders and 20 (24.4%) in the group of nonresponders (P = .025). In multivariate analysis, the only independent predictors of the appearance of ventricular arrhythmias were secondary–prevention device implantation (odds ratio = 4.04; 95% confidence interval, 1.52-10.75; P=.005), absence of echocardiographic super-response (odds ratio=3.81; 95% confidence interval, 1.04-13.93; P=043), QRS >160ms (odds ratio=2.39; 95% confidence interval, 1.00-1.35; P=.049) and treatment with amiodarone (odds ratio=2.47; 95% confidence interval, 1.03-5.91; P=.041).
ConclusionsThe patients classified as super-responders to cardiac resynchronization therapy had a significant reduction in incidence of ventricular arrhythmias by comparison with the other patients. Despite this, arrhythmic episodes do not completely disappear in this subgroup.
Keywords
Cardiac resynchronization therapy (CRT) has been shown to improve quality of life and reduce hospitalization and mortality of patients with severe ventricular dysfunction and wide QRS in the surface electrocardiogram.1–4
The degree of ventricular remodeling with biventricular pacing varies greatly. It has been reported that up to 20% or 30% of patients have a very significant reduction in ventricular volume and near normalization of left ventricular ejection fraction (LVEF). These patients have been termed super-responders (SR) because of their reduced mortality and cardiovascular events, compared with slight responders or nonresponders (NR).5–6
However, the role of CRT in reducing ventricular arrhythmias is debatable and the issue excites much controversy. Some studies confirm that CRT is associated with a reduction in sudden death and ventricular arrhythmias,7–11 principally when there is positive remodeling and a long follow-up. Other studies show no reduction or even an increase in ventricular arrhythmias.12–15 The reduction in ventricular arrhythmias is related to positive ventricular remodeling, improved ejection fraction, and a reduction in ventricular volume, parietal stress, and neurohormonal activity. The increased incidence of ventricular arrhythmias is caused by a change in the activation sequence from the epicardium to the endocardium, with greater transmural dispersion of repolarization, lengthening of the QT interval, and the risk of polymorph arrhythmias of the torsade de pointes type.
The objective of the present study is to analyze and compare the incidence of ventricular arrhythmias in patients receiving CRT with implantable cardioverter-defibrillators (ICDs) in terms of the type of echocardiographic response. In the case of absence or very low incidence of ventricular arrhythmias in patients considered SR, when the time comes to replace the device we might consider substituting a pacemaker for the ICD, with the consequent cost savings-of vital importance nowadays.
METHODSSelection of Patients and DesignWe conducted a retrospective analysis of patients who had undergone CRT-ICD implantation. We included all patients with a CRT-ICD implanted in our center between June 1999 and February 2012. The inclusion criteria were the following: patients with heart failure and in New York Heart Association functional class ≥ II, left ventricular ejection fraction (LVEF) ≤ 35%, QRS ≥120ms, and optimal medical treatment.
The following variables were analyzed: age, sex, New York Heart Association functional class, type of heart disease, previous myocardial infarction, LVEF, QRS width, left ventricular end-systolic diameter and left ventricular end-diastolic diameter, mitral regurgitation grade, primary– or secondary–prevention implantation, presence of atrial fibrillation, type of conduction disturbance, final position of the electrode in 2 different projections (apical, medial or basal in posteroanterior and anterior projection, lateral or posterior in lateral projection), left ventricle electrode threshold, drug treatment for heart failure, antiarrhythmic drug treatment, and presence of diabetes mellitus or chronic kidney failure, which was defined as creatinine clearance < 50 mL/min.
Follow-upFollow-up in ICD outpatient clinics was every 6 months or 1 year. We also used distance-monitoring when the device implanted was equipped with this technology. Furthermore, the devices were examined on visits to the emergency room for any ICD-related symptoms. Three expert electrophysiologists (IFL, JTR and VCU) reviewed and analyzed all episodes of ventricular arrhythmia.
Classification of PatientsWe classified as SR16 those patients with LVEF either at least twice that of the measurement taken at implantation or ≥ 45% at 12 months postimplantation. For patients with < 1-year follow-up, we used data from the last recorded echocardiogram. We classified as responders (R)17 those with LVEF of ≥ 5 points more at 12 months postimplantation than the measurement taken at implantation. For patients with < 1-year follow-up, we used data from the last recorded echocardiogram. All other patients were classified as NR.
EchocardiogramsAll echocardiographic studies were performed with Philips iE33® equipment. All patients underwent echocardiography at implantation and again at 12 months. The following measurements were taken: LVEF using Simpson equation, left ventricular end-systolic diameter, left ventricular end-diastolic diameter, and mitral regurgitation grade. Three expert echocardiographers (SMS, VMP and IGL) reviewed all the studies.
Device ProgrammingWe defined as ventricular arrhythmia any episode detected by the ICD that required antitachycardia pacing or shock treatment. The ICD programming was at the discretion of the physician responsible. However, the standard approach to programming in our center is based on long detection intervals and high cutoff points for tachycardia and fibrillation, which reduces the risk of adopting avoidable or inappropriate therapies.18,19 Furthermore, programming normally involves sequences of antitachycardia pacing in the ventricular tachycardia zone and antitachycardia pacing during or before the shock in the ventricular fibrillation zone.20 We took no account of nonsustained ventricular arrhythmia or what we considered inappropriate therapy.
Statistical AnalysisTo test the normality of the variables, the Kolmogorov-Smirnov statistic was used. Quantitative variables are given as mean (standard deviation) and median [interquartile range] in the case of non-normal distribution. Qualitative variables are expressed as absolute frequencies and percentage. The baseline characteristics in the 3 subgroups of patients were compared using chi-square for categorical variables and analysis of variance for quantitative variables, and the Bonferroni correction was used to adjust for multiple comparisons. The odds ratio (OR) for ventricular arrhythmias was calculated with chi-square. To determine the independent effect of variables on the incidence of ventricular arrhythmias, we constructed a logistic regression model including all the variables with a value of P < .10 in the univariate analysis. We constructed a Kaplan-Meier curve to compare the event-free survival rate by subgroups using the log rank test. We also analyzed mortality by subgroups using the log rank test. Statistical analysis was with SPSS 15.0 for Windows (SPSS Inc.; Chicago, Illinois, United States).
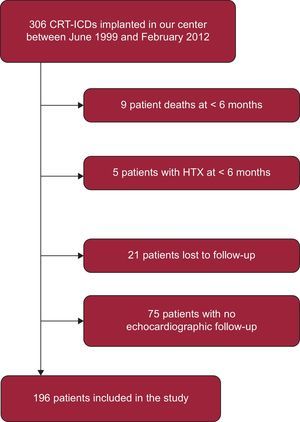
RESULTSA total of 306 patients received a CRT-ICD implant between June 1999 and February 2012. Of these, we excluded 9 patients who died in the first 6 months, 5 patients who underwent heart transplantation in the same period, 21 lost to the follow-up, and 75 with inadequate echocardiographic follow-up (Figure 1). Hence, the study population consisted of 196 patients. We found no significant differences between the groups of patients included and excluded from the analysis in terms of the principle baseline characteristics: age (P = .6), sex (P = .07), New York Heart Association functional class (P = .5), baseline heart disease (P = .08), previous acute myocardial infarction (P = .1), atrial fibrillation (P = .2), secondary–prevention implantation (P = .2), QRS width (P = .5), and initial LVEF (P = .08). However, we did find a higher incidence of left branch bundle block among those patients included in the study than in those excluded (80.1% vs 66%; P = .004).
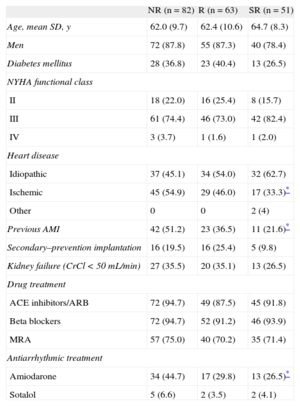
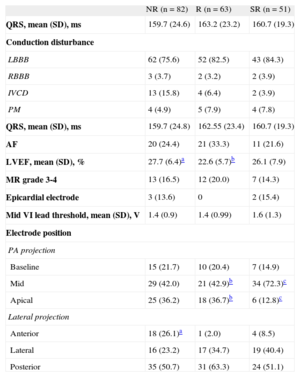
The baseline characteristics of patients in terms of type of echocardiographic response to CRT are in Tables 1 and 2.
Baseline Clinical Characteristics of Patients According to Type of Response to Cardiac Resynchronization Therapy
| NR (n = 82) | R (n = 63) | SR (n = 51) | |
| Age, mean SD, y | 62.0 (9.7) | 62.4 (10.6) | 64.7 (8.3) |
| Men | 72 (87.8) | 55 (87.3) | 40 (78.4) |
| Diabetes mellitus | 28 (36.8) | 23 (40.4) | 13 (26.5) |
| NYHA functional class | |||
| II | 18 (22.0) | 16 (25.4) | 8 (15.7) |
| III | 61 (74.4) | 46 (73.0) | 42 (82.4) |
| IV | 3 (3.7) | 1 (1.6) | 1 (2.0) |
| Heart disease | |||
| Idiopathic | 37 (45.1) | 34 (54.0) | 32 (62.7) |
| Ischemic | 45 (54.9) | 29 (46.0) | 17 (33.3)* |
| Other | 0 | 0 | 2 (4) |
| Previous AMI | 42 (51.2) | 23 (36.5) | 11 (21.6)* |
| Secondary–prevention implantation | 16 (19.5) | 16 (25.4) | 5 (9.8) |
| Kidney failure (CrCl < 50 mL/min) | 27 (35.5) | 20 (35.1) | 13 (26.5) |
| Drug treatment | |||
| ACE inhibitors/ARB | 72 (94.7) | 49 (87.5) | 45 (91.8) |
| Beta blockers | 72 (94.7) | 52 (91.2) | 46 (93.9) |
| MRA | 57 (75.0) | 40 (70.2) | 35 (71.4) |
| Antiarrhythmic treatment | |||
| Amiodarone | 34 (44.7) | 17 (29.8) | 13 (26.5)* |
| Sotalol | 5 (6.6) | 2 (3.5) | 2 (4.1) |
ACE, angiotensin converting enzyme; AMI, acute myocardial infarction; ARB, angiotensin receptor blockers; CrCl: creatinine clearance; MRA, mineralocorticoid receptor antagonists; NR, nonresponders; NYHA, New York Heart Association; R, responders; SD, standard deviation; SR, super-responders.
Data are expressed No. (%) or mean (standard deviation).
Electrocardiographic and Echocardiographic Characteristics Related to Device Implantation in Patients According to Type of Response to Cardiac Resynchronization Therapy
| NR (n = 82) | R (n = 63) | SR (n = 51) | |
| QRS, mean (SD), ms | 159.7 (24.6) | 163.2 (23.2) | 160.7 (19.3) |
| Conduction disturbance | |||
| LBBB | 62 (75.6) | 52 (82.5) | 43 (84.3) |
| RBBB | 3 (3.7) | 2 (3.2) | 2 (3.9) |
| IVCD | 13 (15.8) | 4 (6.4) | 2 (3.9) |
| PM | 4 (4.9) | 5 (7.9) | 4 (7.8) |
| QRS, mean (SD), ms | 159.7 (24.8) | 162.55 (23.4) | 160.7 (19.3) |
| AF | 20 (24.4) | 21 (33.3) | 11 (21.6) |
| LVEF, mean (SD), % | 27.7 (6.4)a | 22.6 (5.7)b | 26.1 (7.9) |
| MR grade 3-4 | 13 (16.5) | 12 (20.0) | 7 (14.3) |
| Epicardial electrode | 3 (13.6) | 0 | 2 (15.4) |
| Mid VI lead threshold, mean (SD), V | 1.4 (0.9) | 1.4 (0.99) | 1.6 (1.3) |
| Electrode position | |||
| PA projection | |||
| Baseline | 15 (21.7) | 10 (20.4) | 7 (14.9) |
| Mid | 29 (42.0) | 21 (42.9)b | 34 (72.3)c |
| Apical | 25 (36.2) | 18 (36.7)b | 6 (12.8)c |
| Lateral projection | |||
| Anterior | 18 (26.1)a | 1 (2.0) | 4 (8.5) |
| Lateral | 16 (23.2) | 17 (34.7) | 19 (40.4) |
| Posterior | 35 (50.7) | 31 (63.3) | 24 (51.1) |
AF, atrial fibrillation; IVCD, intraventricular conduction delay; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NR, nonresponders; PA, posteroanterior; PM, pacemaker rhythm; R, responders; RBBB, right bundle branch block; SD, standard deviation; SR, super-responders.
Data are expressed No. (%) or mean (standard deviation).
Fewer SR patients had ischemic heart disease and previous acute myocardial infarction compared with NR patients. Fewer patients were receiving amiodarone treatment in the SR group than in the NR group. There were no differences between groups in terms of the administration of standard drugs for the treatment of heart failure or presence of chronic kidney failure or diabetes mellitus. The apical position of the left ventricle electrode appeared more frequently in the NR and R groups than in the SR group. Furthermore, in NR patients, the anterior position was more frequent than in R patients.
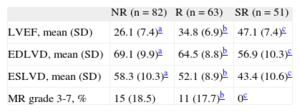
Of the 196 patients enrolled, we classified 51 as SR (26%), 63 as R (32%), and 82 as NR (42%) on the basis of the echocardiographic criteria defined above. The SR patients showed a statistically significant improvement of all echocardiographic parameters (LVEF, left ventricular end-systolic diameter, and left ventricular end-diastolic diameter) by comparison with the R and NR patients (Table 3).
Comparison of Echocardiographic Parameters Between Super-responders, Responders and Nonresponders in the Follow-up
| NR (n = 82) | R (n = 63) | SR (n = 51) | |
| LVEF, mean (SD) | 26.1 (7.4)a | 34.8 (6.9)b | 47.1 (7.4)c |
| EDLVD, mean (SD) | 69.1 (9.9)a | 64.5 (8.8)b | 56.9 (10.3)c |
| ESLVD, mean (SD) | 58.3 (10.3)a | 52.1 (8.9)b | 43.4 (10.6)c |
| MR grade 3-7, % | 15 (18.5) | 11 (17.7)b | 0c |
EDLVD, left ventricular end-diastolic diameter; ESLVD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction MR, mitral regurgitation; NR, nonresponders; R, responders; SD, standard deviation; SR, super-responders.
Data are expressed No. (%) or mean (standard deviation).
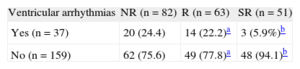
After a median follow-up of 30.1 [18.0-55.1] months, 37 patients (18.8%) had ventricular arrhythmias: 3 (5.9%) in the SR group, 14 (22.2%) in the R group, and 20 (24.4%) in the NR group (Table 4).
Incidence of ventricular arrhythmias according to the type of echocardiographic response
| Ventricular arrhythmias | NR (n = 82) | R (n = 63) | SR (n = 51) |
| Yes (n = 37) | 20 (24.4) | 14 (22.2)a | 3 (5.9%)b |
| No (n = 159) | 62 (75.6) | 49 (77.8)a | 48 (94.1)b |
NR, nonresponders; R, responders; SR, super-responders.
Data are expressed No. (%).
The probability of having a ventricular arrhythmia in the R group by comparison with the SR group was OR = 4.6 (95% confidence interval [95%CI], 1.2-16.9; P = .015). In other words, there was a 78.2% reduction in the risk of presenting ventricular arrhythmias in the SR group by comparison with the R group. In the NR group, the risk was OR = 5.2 (95%CI, 1.5-18.4; P = .006) by comparison with the SR group. This represents an 80.8% reduction in the risk of ventricular arrhythmias in the SR group. There were no significant differences in the risk of arrhythmias between the R and NR groups (OR = 1.1; 95%CI 0.7-1.7; P = .8).
During the follow-up, 40 patients died (20.4%). There were no significant differences between the subgroups in terms of echocardiographic response (P = .92).
Since we found no statistically significant differences between the R and NR groups in terms of the recurrence of arrhythmias, these groups were joined into one (non–SR) for the arrhythmia-free survival curve and univariate and multivariate analyses.
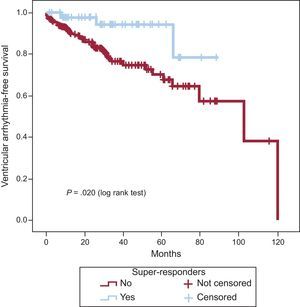
The Kaplan-Meier curve analysis for cumulative probability of ventricular arrhythmias (Figure 2) showed that SR patients had less risk of arrhythmic events (P = .020) than the other two groups analyzed together.
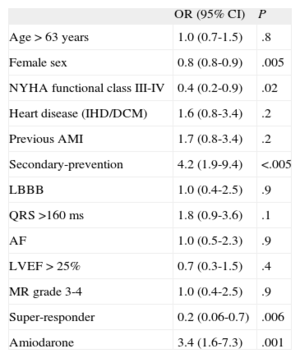
In univariate analysis (Table 5), female sex (OR = 0.8; 95%CI, 0.8-0.9), echocardiographic super-response (OR = 0.20; 95% CI, 0.06-0.70) and New York Heart Association functional class III-IV (OR = 0.4; 95%CI, 0.2-0.9) were the only statistically significant variables that protected against ventricular arrhythmias. In contrast, secondary–prevention ICD implantation (OR = 4.2; 95%CI, 1.9-9.4) and treatment with amiodarone (OR = 3.4; 95%CI, 1.6-7.3) were associated with greater risk of ventricular tachycardia during follow-up.
Univariate analysis of Prediction of Ventricular Arrhythmias According to Clinical Variables
| OR (95% CI) | P | |
| Age > 63 years | 1.0 (0.7-1.5) | .8 |
| Female sex | 0.8 (0.8-0.9) | .005 |
| NYHA functional class III-IV | 0.4 (0.2-0.9) | .02 |
| Heart disease (IHD/DCM) | 1.6 (0.8-3.4) | .2 |
| Previous AMI | 1.7 (0.8-3.4) | .2 |
| Secondary-prevention | 4.2 (1.9-9.4) | <.005 |
| LBBB | 1.0 (0.4-2.5) | .9 |
| QRS >160 ms | 1.8 (0.9-3.6) | .1 |
| AF | 1.0 (0.5-2.3) | .9 |
| LVEF > 25% | 0.7 (0.3-1.5) | .4 |
| MR grade 3-4 | 1.0 (0.4-2.5) | .9 |
| Super-responder | 0.2 (0.06-0.7) | .006 |
| Amiodarone | 3.4 (1.6-7.3) | .001 |
95%CI, 95% confidence interval; AF, atrial fibrillation; AMI, acute myocardial infarction; DCM, dilated cardiomyopathy; IHD, ischemic heart disease; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NYHA, New York Heart Association; OR, odds ratio.
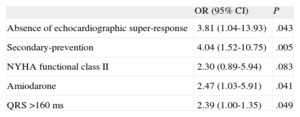
Finally, we conducted multivariate analysis. We could not include sex in the multivariate analysis because none of the women had recurrence of ventricular arrhythmia in the follow-up. In multivariate analysis, the lack of super-response to CRT (OR = 3.81; 95%CI, 1.04-13.93; P = .043), secondary–prevention device implantation (OR = 4.04; 95%CI, 1.52-10.75; P = .005), amiodarone treatment (OR = 2.47; 95%CI, 1.03-5.91; P = .041), and QRS >160ms (OR = 2.39; 95%CI, 1.00-1.35; P = .049) were the only independent predictors of ventricular arrhythmias (Table 6).
Multivariate analysis of prediction of ventricular arrhythmias
| OR (95% CI) | P | |
| Absence of echocardiographic super-response | 3.81 (1.04-13.93) | .043 |
| Secondary-prevention | 4.04 (1.52-10.75) | .005 |
| NYHA functional class II | 2.30 (0.89-5.94) | .083 |
| Amiodarone | 2.47 (1.03-5.91) | .041 |
| QRS >160 ms | 2.39 (1.00-1.35) | .049 |
95%CI, 95% confidence interval; NYHA, New York Heart Association; OR, odds ratio.
The objective of this study was to compare the incidence of ventricular arrhythmias in patients receiving CRT with ICD implantations according to their echocardiographic response in terms of ventricular remodeling.
Currently, decision-making prior to ICD implantation is principally based on LVEF, and LVEF ≤ 35% is generally accepted as the indication for implantation.21 Consequently, in patients with CRT-ICD, if LVEF has improved when ICD replacement is necessary, especially in SR patients, it may be worth considering substituting the ICD with a CRT-pacemaker, with the consequent saving in costs.
In SR-defined as patients whose LVEF is ≤ 45% or at least twice their baseline measurement-we found that incidence of ventricular arrhythmias was 5.9% after a median follow-up of 30.1 months [18.0-55.1 months].
The mechanisms of association between heart failure, left ventricular dilatation, and ventricular arrhythmias are complex. Empirical evidence indicates the degree of inverse remodeling is associated with the presence of ventricular arrhythmias. The Survival and Ventricular Enlargement, study reported that changes in ventricle size and function predicted ventricular extrasystole and ventricular tachycardia.22 Treatment with drugs like beta blockers and angiotensin-converting enzyme inhibitors also reduces the incidence of ventricular arrhythmias and sudden death.23
The role CRT plays in reducing ventricular arrhythmias is under debate. The CONTAK-CD study, which compared CRT-ICD with conventional ICD use in 490 patients with heart failure and in New York Heart Association functional class II-IV, found the incidence of appropriate shock after 6 months’ follow-up was similar in both groups: 15% in CRT patients vs 16% in the conventional ICD group.24
Similarly, the MIRACLE ICD study25 found no differences between patients with CRT and patients with conventional ICD in terms of appropriate shock or antitachycardia pacing therapy. This study included 369 patients and follow-up was relatively short (6 months).
Benefits have been obtained in more long-term follow-up. In the extended CARE-HF study, with a 3-year follow-up, CRT therapy without ICD was associated with a fall in sudden death (4.3% per year in those without CRT vs 2.5% per year in those with CRT).7 It seems that with CRT, the reduction in arrhythmias occurs in the mid- or long-term. Analyzing the Kaplan-Meier curves, no separation appears until after 2 years’ follow-up.
Theoretically, the reduction in ventricular arrhythmias due to CRT occurs because of improved cardiac remodeling, reduced myocardial ischemia and parietal stress, and a favorable change in the patient's neurohormonal status.26 Moreover, ventricular arrhythmias are caused by re-entry in areas of myocardial fibrosis with slow conduction. Left ventricle pacing can stimulate these zones early or modify the depolarization vector so that the incidence of ventricular tachycardia is reduced.27 Hence, the site of the left ventricle lead is of vital importance.
Notwithstanding, the degree of ventricular remodeling should predict the more important ventricular events. In 2009, Markowitz et al8 reported a reduction in the incidence of ventricular extrasystole, nonsustained ventricular tachycardia, and episodes of ventricular tachycardia or ventricular fibrillation in R patients.
Subsequently, Shahrzad et al10 reported that incidence of ventricular arrhythmias in patients receiving CRT-ICD fell in R patients-defined as patients with an improvement in LVEF of ≥5 points or reduction in end-diastolic diameter of at least 10%.
In a MADIT-CRT substudy,9 CRT-group patients were divided into 2 subgroups according to the degree of response (reduction of left ventricular end-systolic volume > 25% vs < 25%). The probability of having ventricular arrhythmia after 2 years’ follow-up was lower in the R group (12%) than in patients with a conventional ICD (21%) or in NR patients (28%).
In our study, the incidence of ventricular arrhythmias in the SR group (5.9%) was lower than in the R (22.2%) and the NR (24.4%) groups. There was a significant reduction in the incidence of ventricular arrhythmia between SR and the other groups, with no differences between R and NR. Therefore, only patients with a highly significant degree of echocardiographic remodeling after CRT had a clear reduction in the risk of recurrent ventricular arrhythmias in the follow-up. This could explain the heterogeneity of results in studies conducted to date, which generally use less restrictive cutoff points for ventricular remodeling to classify patients as R.
The presence of systolic dysfunction is associated with the risk of ventricular arrhythmias and sudden death. Patients classified as SR to CRT represent a special subgroup who may be in less advanced stages of heart disease and have better conserved ventricular geometry. In these patients, inverse ventricular remodeling and recovery of systolic function postimplantation are also associated with a reduced risk of arrhythmias-even though this risk does not completely disappear. Positive remodeling may occur, with improved ejection fraction that might even return to normal. However, areas of fibrosis or slow conduction can persist, which may be potential or real circuits for the development of ventricular arrhythmias.
In our study, we found 5.9% incidence of ventricular arrhythmia after a median follow-up of 30.1 [18.0-55.1] months despite super-response to CRT, which suggests that replacing the ICD with a pacemaker, when device replacement is necessary, may be unwise.
LimitationsThis retrospective observational study was conducted in a single center over a very long period of time (13 years), so it was subject to uncontrolled factors.
We found treatment with amiodarone to be among the factors predicting ventricular arrhythmia. Clearly there is a bias, given that patients who had ventricular arrhythmias received this treatment more frequently.
We found no differences between the subgroups in terms of mortality. Aside from the fact that this was not the objective of the study, the follow-up may have been too short for CRT to show differences in survival.
CONCLUSIONSWe identified four independent predictors of ventricular arrhythmias in our follow-up of patients with CRT: QRS > 160ms, secondary–prevention ICD implantation, treatment with amiodarone, and echocardiographic classification other than SR.
The SR patients had a clear reduction in incidence of ventricular arrhythmias by comparison with the other groups. Even so, a not inconsiderable percentage of SR patients continue to have arrhythmic episodes during follow-up. Therefore, for this group of patients, it seems unreasonable to cease ICD use when device replacement is due.
CONFLICTS OF INTERESTNone declared.