We aimed to assess and compare the effect of digoxin on clinical outcomes in patients with atrial fibrillation vs those under beta-blockers or none of these drugs.
MethodsAFBAR is a prospective registry study carried out by a team of primary care physicians (n=777 patients). Primary endpoints were survival, survival free of admission due to any cause, and survival free of admission due to cardiovascular causes. The mean follow up was 2.9 years. Four groups were analyzed: patients receiving digoxin, beta-blockers, or digoxin plus beta-blockers, and patients receiving none of these drugs.
ResultsOverall, 212 patients (27.28%) received digoxin as the only heart control strategy, 184 received beta-blockers (23.68%), 58 (7.46%) were administered both, and 323 (41.57%) received none of these drugs. Digoxin was not associated with all-cause mortality (estimated hazard ratio=1.42; 95% confidence interval, 0.77-2.60; P=.2), admission due to any cause (estimated hazard ratio=1.03; 95% confidence interval, 0.710-1.498; P=.8), or admission due to cardiovascular causes (estimated hazard ratio=1.193; 95% confidence interval, 0.725-1.965; P=.4). No association was found between digoxin use and all-cause mortality, admission due to any cause, or admission due to cardiovascular causes in patients without heart failure. There was no interaction between digoxin use and sex in all-cause mortality or in survival free of admission due to any cause. However, an association was found between sex and admission due to cardiovascular causes.
ConclusionsDigoxin was not associated with increased all-cause mortality, survival free of admission due to any cause, or admission due to cardiovascular causes, regardless of underlying heart failure.
Keywords
Digitalis is the oldest compound in cardiovascular medicine that continues to be used in contemporary clinical practice.1,2 However, evidence supporting its use is nowadays controverial, mainly for heart rate control in patients with atrial fibrillation (AF).3,4 With the current development and introduction of life-saving therapies (beta-blockers [BB], angiotensin receptor blockers, aldosterone blockers) and safety concerns in recent reports, evidence in this field is warranted to determine its continued use.
The aim of our study was to assess and compare the effect of digoxin on clinical outcomes in patients with AF vs those under BB or none of these drugs in the general population and in patients with a diagnosis of heart failure (HF).
METHODSStudy PopulationThe AFBAR (Atrial Fibrillation in the BARrbanza area) was a registry that has been previously described in detail.5,6 Briefly, AFBAR was carried out by a team of 35 primary care physicians in a single health area in Galicia, north-west Spain. AFBAR aimed to describe the natural history of AF in an unselected population treated by primary care services and at the discretion of their treating physicians. Each physician enrolled all his or her patients with AF aged > 18 years during a 3-month period (from January 2008 to April 2008). All patients signed an informed consent form. The study protocol was in line with the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the human research committee of the institution. The patients’ demographic and clinical data, such as previous cardiovascular events and other comorbidities, treatment, and AF complications during follow up, were ascertained from a clinical interview and hospital records. AFBAR was performed in 777 patients. Patients were classified according to their type of AF: first episode or new-onset AF; recurrent AF (≥2 episodes of AF); paroxysmal AF (recurrent AF with spontaneous reversion to sinus rhythm); persistent AF (recurrent AF with an arrhythmic event of ≥ 7 days, or AF requiring electrical or pharmacological cardioversion to restore sinus rhythm), and permanent longstanding AF (established AF (1 year) or AF with rate control managed by the primary care physician). Persistent AF was termed ‘permanent’ once AF was established and pharmacological or electrical cardioversion had either failed or had been discontinued for clinical or echocardiographic reasons. Heart failure was defined as symptoms of HF (at rest or during exercise) and evidence of cardiac dysfunction (systolic and/or diastolic) and, in patients in whom the diagnosis was in doubt, response to treatment for HF.
The primary endpoints were survival, morbidity (survival free of admission due to any cause) and cardiovascular morbidity (survival free of admission due to cardiovascular causes). For this purpose, 4 groups were analyzed independently: patients under digoxin (group 1), those receiving BB (group 2), those receiving digoxin plus BB (group 3), and those receiving none of these drugs (group 4).
Statistical AnalysisDescriptive data are reported as frequencies, mean (standard deviation), or as the median, where appropriate. For group comparisons, 2-sided Student t tests for independent samples were used for continuous variables and the chi-squared test was used for binary variables. One-way analysis of variance was used identify significant differences among study groups (Figure 1). After verifying proportionality assumptions, multivariate Cox proportional hazards models were used to assess the impact of digoxin on all-cause mortality, morbidity and cardiovascular morbidity, while controlling for multiple covariates (age, body mass index, diabetes mellitus, hypertension, dyslipidemia, ischemic heart disease, smoking, HF, previous stroke, left ventricular ejection fraction, renal insufficiency, BB, calcium channel blockers, and antiarrhythmic drugs). Digoxin use was assessed at a fixed time point only. Confounding and interaction between digoxin use and HF and between digoxin use and sex was also evaluated. All statistics were computed with SPSS software (SPSS Inc., Chicago, IL, United States). All probability values were 2-sided, with P values of<.05 considered significant.
RESULTSThe mean age was 74.9 years (9.3 years) and 365 of the patients were women, representing 46.9% of the study cohort. The demographic findings are summarized in the Table. Briefly, most of the sample was categorized as having permanent AF (n=529), 96 of them with a clinical diagnosis of HF. The mean time since onset of AF was 6.1 years. In 588 patients (75.7%), an echocardiogram had been performed <12 months prior to enrolment.
Baseline Characteristics of AFBAR Registry Population (N=777)
| Digoxin (n=212) | BB (n=184) | Digoxin + BB (n=58) | None (n=323) | Total (n=777) | P | |
| Age, mean (SD), y | 76.9 (8.4) | 72.7 (9.3) | 74.4 (10.7) | 76.7 (9.2) | 74.8 (9.3) | <.001 |
| AF duration, mean (SD), y | 7.9 (5.7) | 5.8 (4.9) | 6.3 (4.3) | 5.2 (4.8) | 6.2 (5.2) | <.001 |
| Female/Male, % | 53.8/46.2 | 47.8/52.2 | 53.4/46.6 | 40.7/59.3 | 46.9/53.1 | .01 |
| Hemoglobin, mean (SD), g/dL | 13.75 (1.60) | 13.90 (1.50) | 13.60 (1.60) | 13.80 (1.50) | 13.80 (1.50) | .6 |
| Creatinine, mean (SD), mg/dL | 1.04 (0.24) | 1.05 (0.28) | 1.03 (0.22) | 1.06 (0.30) | 1.05 (0.27) | .03 |
| Glycated hemoglobin | 6.8 (1.4) | 6.3 (1.4) | 7.5 (1.8) | 6.6 (1.2) | 6.7 (1.4) | .01 |
| BMI, mean (SD) | 29.7 (4.8) | 30.6 (4.7) | 30.4 (4.5) | 30.0 (4.9) | 30.1 (4.8) | .3 |
| Abdominal circumference, mean (SD), cm | 98.60 (12.10) | 101.60 (11.50) | 99.73 (12.70) | 100.80 (12.40) | 100.30 (12.20) | .08 |
| Hypertension, % | 77.8 | 75.5 | 79.3 | 75.6 | 76.5 | .8 |
| Diabetes mellitus, % | 28.8 | 22.3 | 37.9 | 21 | 24.7 | .01 |
| Ischemic heart disease, % | 17.0 | 25.5 | 17.2 | 13.3 | 17.5 | .06 |
| Sedentary lifestyle, % | 59.9 | 47.3 | 48.3 | 45.4 | 50 | <.01 |
| Heart failure, % | 19.8 | 11.4 | 22.4 | 6.2 | 12.3 | <.01 |
| Prosthesis, % | 7.5 | 1.6 | 3.4 | 1.9 | 3.5 | <.01 |
| Valvulopathy, % | 42.5 | 29.9 | 55.2 | 22.5 | 32.1 | <.01 |
| Renal insufficiency, % | 9.9 | 9.8 | 10.3 | 9.3 | 9.6 | .9 |
| COPD, % | 25.5 | 10.90 | 10.3 | 19.1 | 18.3 | .01 |
| Stroke, % | 5.2 | 3.8 | 1.7 | 4.6 | 4.4 | .6 |
| LVEF, % | 59.85 (10.9) | 58.16 (13.74) | 54.99 (13.72) | 62.40 (10.26) | 59.97 (11.74) | .01 |
| Previous admission, No. (mean [SD]) | 47 (0.31 [0.7]) | 42 (0.28 [0.5]) | 14 (0.34 [0.7]) | 63 (0.25 [0.5]) | 166 (0.28 [0.6]) | .5 |
| Cardiovascular admission, No. (mean [SD]) | 20 (0.12 [0.5]) | 30 (0.18 [0.4]) | 7 (0.49 [0.5]) | 35 (0.13 [0.4]) | 92 (0.14 [0.4]) | .4 |
AF, atrial fibrillation; BB, beta-blockers; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; SD, standard deviation.
Unless otherwise indicated, the data are expressed No. (%).
Among patients under the strategy of “rate control”, overall, 212 patients (27.28%) received digoxin as the only rate control strategy (group 1), 184 received BB (23.68%) (group 2), 58 (7.46%) were administered both (group 3), and 323 (41.57%) received none of these drugs (group 4). Differences among groups are described in the Table. Notably, patients under digoxin therapy were older and had a higher rate of HF, diabetes mellitus, sedentariness, and chronic obstructive pulmonary disease.
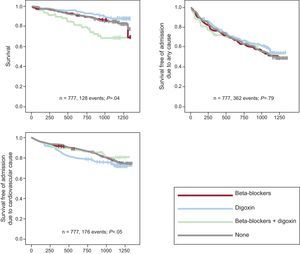
Results for all AFBAR PatientsOf the 777 patients sampled, 128 (16.5%) died during the study period (14.4% without HF and 30.5% with HF). The mean follow up was 1 067.3 days (95% confidence interval [95%CI], 964.6-1170.1); digoxin was not associated with all-cause mortality (estimated hazard ratio [EHR]=1.42; 95%CI, 0.77–2.60; P=.2; C-statistic=0.74). Specifically, in the 212 patients under digoxin therapy (group 1), there were 35 deaths (16%); in patients receiving BB (group 2), there were 22 deaths out of 184 patients (11.9%). There were no differences between these 2 groups (P=.2). However, when patients under BB plus digoxin (group 3), with 18 deaths out of 58 patients (31%), and those not treated with BB or digoxin (group 4), with 53 deaths out of 323 patients (16%), were taken into account, differences between groups became significant (P=.04) (Figure 1).
Digoxin was not associated with admission due to any cause (EHR=1.03; 95%CI, 0.710-1.498; P=.8; C-statistic=0.56). There were a total of 362 events (46.6%), with no differences among groups: 101 of 212 (47.6%) in group 1, 81 of 184 (44%) in group 2, 26 of 58 (44.82%) in group 3, and 154 of 323 (47.64%) in group 4. Differences were not significant when only those patients assigned to digoxin or BB were analyzed (P=.3).
With regard to cardiovascular morbidity (survival free of admission due to cardiovascular causes), there were a total of 176 events (22.65%), again without differences among the 4 groups: 47 of 212 (22.17%) in group 1, 49 of 184 (26.63%) in group 2, 10 of 58 (17.24%) in group 3, and 70 of 323 (21.67%) in group 4. There were no significant differences when patients under digoxin therapy and BB were analyzed independently (P=.3). Similarly, digoxin was not associated with an increase in cardiovascular admission due to cardiovascular causes (EHR=1.193; 95% CI, 0.725–1.965; P=.4; C-statistic=0.68).
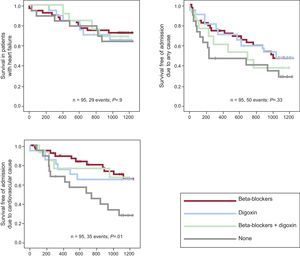
Results for all AFBAR Patients With Heart FailureOf the 95 patients with HF, 29 (30.52%) died during the study period. Digoxin was not associated with admission due to any cause (EHR=0.94; 95%CI, 0.20-4.41; P=.9). There were no differences in overall survival among patients taking digoxin or BB or among the 4 categories previously mentioned: group 1: 11 of 41 (26.83%); group 2: 7 of 21 (33.33%); group 3: 4 of 13 (30.77%); group 4: 7 of 20 (35%). Analysis of morbidity showed that there were a total of 50 events in 95 individuals (52.63%): 19 of 41 (46.34%) in group 1, 10 of 21 (47.62%) group 2, 8 of 13 in group 3 (61.53%), and 13 of 20 (65%) in group 4 (Figure 2). The adjusted EHR for digoxin exposure was 1.6; (95%CI, 0.9-2.9; P=.9). Finally, concerning cardiovascular morbidity, digoxin was not associated with an increase in cardiovascular admission due to cardiovascular causes (EHR=0.78; 95%CI, 0.49-1.23; P=.4). There were no differences between patients under digoxin and those under BB therapy (P=.5) but statistically significant differences were found when the patients were divided into the 4 previously mentioned groups, with a total number of events of 35 of 95 patients (36.84%): group 1: 11 of 41 (26.83%); group 2; 7 of 21 (33.33%); group 3: 4 of 13 (30.77%), and group 4: 13 of 20 (65%) (P=.01).
A fully adjusted Cox proportional hazards model with a term for the interaction between digoxin use and HF yielded no confounding or interaction effects for all-cause mortality, survival free of admission due to any cause, and survival free of admission due to cardiovascular causes.
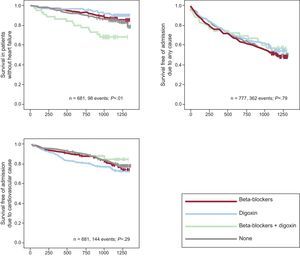
Results for all AFBAR Patients Without Heart FailureThe analysis was also conducted for patients without a clinical diagnosis of HF. Digoxin was not associated with all-cause mortality (EHR=0.94; 95%CI, 0.20–4.41, P=.9). Differences between groups 1 and 2 in overall survival were not significant (P=.2), but there were differences among the 4 groups (P=.01). Summarizing, there were a total of 98 deaths in 681 patients: 23 of 170 (13.53%) in group 1; 15 of 163 in group 2 (9.20%); 14 of 45 in group 3 (31.11%), and 46 of 303 group 4 (15.18%). There were no differences in morbidity among patients in the 4 groups: 311 of 681 (45.67%), 81 of 170 (47.65%), 71 of 113 (62.83%), 18 of 45 (40%), 141 of 303 (46.53%) in groups 1, 2, 3 and 4, respectively (Figure 3). The adjusted EHR for digoxin exposure was 1.02 (95%CI, 0.37-2.81; P=.9). Concerning cardiovascular mortality, there were 141 events in 681 patients without HF (20.7%); 36 events in 170 patients (21.17%) in group 1; 42 in 163 patients in group 2 (25.76%), 6 in 45 patients in group 3 (13.33%), and 57 of 303 patients in group 4 (18.81%), with no association between digoxin and admission due to cardiovascular causes (EHR=0.64; 95%CI, 0.22-1.86, P=.4).
Results by SexDigoxin therapy was more frequent in women than in men (40% vs 30.2%, P=.06). There were 64 deaths in 365 women (17.53%) vs 64 in 412 men (15.53%), with no differences among groups (P =.4); 158 women were admitted to hospital due to any cause from a total of 365 (43.28%) vs 204 of 412 men (49.51%) (P =.1); finally, 73 women out of 365 (20%) were admitted due to cardiovascular causes vs 103 men out of 412 (25%) (P =.1). Sex showed no multivariate-adjusted associations with all-cause mortality (HR=1.22; 95%CI, 0.53–2.78; P=.6) or admissions due to any cause (HR=0.81; 95%CI, 0.52–1.27; P=.3), but there was an association between sex and admission due to cardiovascular causes. A fully adjusted Cox proportional hazards model with a term for the interaction between digoxin use and sex showed a significant interaction between sex and digoxin for admission due to cardiovascular causes (P=.013). The HR for digoxin in the adjusted model without the interaction term was 1.03 (95%CI, 0.61-1.76) and was 0.83 (95%CI, 0.5-1.4) for sex. Taking into account the interaction between these 2 variables, the HR for digoxin use in women was 2.56 (95%CI, 1.1-6.1) and that for men was 0.31 (95%CI, 0.1-0.9).
DISCUSSIONIn this prospective registry performed in patients with AF in a single health area, digoxin was not associated with increased all-cause mortality, survival free of admission due to any cause and survival due to cardiovascular causes, regardless of the presence or absence of underlying HF (despite higher risk in patients with HF). Finally, there was no interaction between digoxin use and sex in either all-cause mortality or survival free of admission due to any cause. However, sex acted as a confounding and interaction variable when we considered admission due to cardiovascular causes.
Digitalis is the oldest compound in cardiovascular medicine that continues to be used in contemporary clinical practice.1,2 While it has beneficial effects in HF and can reduce resting heart rate in AF,6,7 some reports have indicated that its use may be an independent risk factor for death.8 This is particularly relevant because other safe and inexpensive alternatives such as BB or calcium blockers are readily available. Some studies have recently been published in this field. The RIKS-HIA9 study and post hoc AFFIRM10 study showed increased mortality among digoxin-treated patients. The first study, RIKS-HIA, examined 1-year outcomes in patients on digoxin with AF, congestive HF, or both, by comparing them with a matched group of patients who were not receiving digoxin.8 Overall mortality was significantly higher in the 4426 digoxin-treated patients with AF and no history of HF than in 16587 controls at discharge (EHR=1.42; 95%CI, 1.29–1.56). Importantly, no such difference was seen in patients with AF and HF, or in patients with HF but without AF. In the AFBAR study, the EHR matched that described in RIKS-HIA, although with wider confidence interval. We cannot rule out the possibility that a larger sample size could have translated into narrower CI. However, some differences are notable between the 2 studies. First, RISK-HIA was performed in an intensive care setting, hampering extrapolation of the results. Secondly, this trial only analyzed total mortality, based on a national survey, and finally the follow up was 1 year vs the mean 2.9 years in AFBAR.
The second study, a recent substudy of AFFIRM10, reported that in patients with AF, digoxin was associated with increased all-cause mortality after controlling for comorbidities and propensity scores, regardless of sex and the presence or absence of underlying HF. Hence, all-cause mortality was 41% higher in patients on digoxin, which discouraged rate control with digoxin as a single first line agent. The SCAF (Stockholm Cohort of Atrial Fibrillation) trial,8 showed that digoxin is mainly given to an elderly and frailer subset of patients with AF; moreover, when these and other differences in patient characteristics are accounted for, digoxin use appears to be neutral for long-term mortality and major cardiovascular events in patients with AF. Consistently, results from the AFBAR study also showed that digoxin is prescribed in high risk patients, even though it appears to have a neutral effect on long-term mortality, after accounting for age, comorbidity, and other patient characteristics. The analysis was consistent when it was performed over the entire cohort (those patients under digoxin, BB, both, or none of these drugs) and when they were divided into those under digoxin or BB alone. However, in terms of overall survival, the group under BB plus digoxin showed a trend toward a worse outcome than those under BB, digoxin or none of these drugs (Figure 1A). Although no final conclusions can be drawn because of the small number of patients (n=58), this finding is important, because it has been postulated that BB could attenuate the neurohormonal effects of digoxin. However, when patients were divided into those with or without HF, there were no significant differences in survival between groups. Moreover, the worse outcome could be partly explained by the greater frailty in this subgroup of patients (higher rate of diabetes mellitus and HF). We believe that this question deserves further investigation to refute or confirm any kind of causality.
Some differences should be highlighted when comparing the AFBAR study with the original AFFIRM study.11 For instance, the AFBAR population was older than that described in AFFIRM (74.8 years vs 69.7 years), and had a higher percentage of women (46.9% vs 39.3%) and hypertension (76.58% vs 70.8%). Coronary artery disease was the predominant cardiac diagnosis in 17.5% of the patients in AFBAR vs 26.1% in AFFIRM. Digoxin was used as monotherapy for rate control in 34.7% of patients in AFBAR vs 17% in AFFIRM. The rate of renal disease was 9.6% in AFBAR and around 5.5% in AFFIRM. The rate of diabetes mellitus was not specified in AFFIRM, but was considerable high in our study population (26.5%). Finally, in AFFIRM, the association of digoxin use with mortality was evaluated by treating digoxin as a time-dependent covariate in a Cox proportional hazard model. By using digoxin as a time-dependent covariate, patients changed from being in the ‘on-digoxin’ group to the ‘not on-digoxin’ group if their medication use changed over time in the study, and their associated time at risk for death contributed to each respective group.12 In contrast, in the AFBAR study, digoxin use was assessed at a fixed time point only, at the time of inclusion. These reasons could explain the differences found in survival values (16.5% in AFFIRM vs 11.9% in AFBAR) during a similar follow up period (around 3 years in AFBAR and 3.5 years in AFFIRM).
On the other hand, comparison between “Lenient versus strict rate control in patients with atrial fibrillation” (RACE II)13 showed that, compared with strict rate control, lenient rate control was not inferior in terms of major clinical events. This could have represented a bias regardless of the drug selected, both in the AFFIRM and AFBAR studies. Unfortunately, strict vs lenient control was not specifically compared in AFFIRM, although a heart rate >100bpm was found to affect all-cause and cardiovascular mortality (EHR=2.92; 95%CI, 2.21–3.85; P=.0001 and EHR=2.31; 95%CI, 1.53–3.50; P=.0001 respectively). Heart rate was not available in our database, thus differences adjusted for heart rate could not be assessed in our study population.
With regards to patients with HF, former studies with digoxin in patients with chronic HF reported improvements in left ventricular ejection fraction,14–17 HF symptoms,18,19 and exercise performance.13,14 Although the Digitalis Investigation Group (DIG) study20 excluded patients with AF, it was the largest trial that examined the safety of digoxin in patients with HF. Patients were randomized to digoxin vs placebo; digoxin was found to have a neutral effect on all-cause mortality (EHR=0.99; 95%CI, 0.91–1.07; P=.80) but reduced the rate of hospitalizations for HF. Further analysis of the DIG trial data demonstrated that the beneficial effect of digoxin only applied to patients in sinus rhythm with low serum digoxin drug levels (0.9 ng/mL). Serum digoxin concentrations were frequently monitored in the DIG trial, which is important, because, as known, positive inotropic and neurohormonal effects are attained with low plasma drug concentrations and, as reported by the DIG trial, patients with higher digoxin levels had worse outcomes.20 Dhaliwal et al,21 in a cohort of HF patients (n=347, 155 of them treated with digoxin), failed to show a reduction in HF hospitalizations or to show any benefit in subgroups of patients with severe left ventricular systolic dysfunction with left ventricular ejection fraction < 25% or New York Heart Association class III or IV. Notably, this study population was older, with a higher proportion of patients with New York Heart Association class III or IV HF, and more comorbid medical conditions, particularly diabetes and hypertension. In the AFBAR study, survival, morbidity, and cardiovascular mortality were not higher in those patients with a clinical diagnosis of HF assigned to digoxin. Of the 95 patients with HF, 29 (30.5%) died during follow-up. Digoxin was not associated with all-cause mortality, admission due to any cause, or admission due to cardiovascular causes. Consistent with the current evidence, those patients with HF without BB had a significantly worse outcome (in terms of hospital admission due to cardiovascular causes) than those under BB, but also than those under digoxin plus BB.
Finally, in our study, we found no sex interaction with digoxin therapy for all-cause mortality or survival free of admission due to any cause. Post hoc analysis of the DIG indicated that digoxin, when used in the treatment of HF, may increase mortality by approximately 20% in women but not in men.22 Nevertheless, this finding has not been confirmed in other observational studies. For instance, a study conducted using the Health Information Network population database,23 which aimed to study the impact of digoxin exposure on mortality in men and women with a diagnosis of HF (n=10 808 women), showed the absence of a large interaction between digoxin use and sex affecting mortality; consistent with the AFBAR and the Health Improvement Network Database are 2 prior observational studies that also found no interaction between digoxin and sex.23,24 Because the finding of an interaction in the DIG trial was the result of a post hoc analysis, it could conceivably be a type 1 error (false positive).23 However, the DIG trial results may also be correct, and the observational results may be biased by unmeasured confounders that affected the interaction analysis. Interestingly, in our study population, sex showed an interaction and was associated with admission due to cardiovascular causes. Although this finding could be due to legitimate differences in prescribing practices in male and female populations with HF,23,24 we believe that this question deserves further investigation.
LimitationsA major limitation of this study is the lack of randomization to digoxin therapy; however, since there are other means to achieve rate control and because of the lack of commercial interest, it would be difficult to carry out a randomized trial with digoxin use for rate control in AF.
Subgroup analyses are exploratory and are inherently limited by smaller sample sizes. Hence, these analyses should be interpreted with caution.
The accuracy of clinical classifications of death (in particular, of cardiovascular or arrhythmic death) is limited. As previously mentioned, another limitation is that individual digoxin doses were not available in the data set; however, the maximal dose during data collection was 0.25mg/day. We cannot, therefore, assess whether serum digoxin levels are predictive of mortality outcomes.
Heart rate values were not collected in the database or in most of the large studies mentioned in the discussion section. This is an important variable that might be worth taking into account in future studies.
Another additional limitation is that the strategy selected (rate vs rhythm control) was not collected in the database and consequently outcome could not be adjusted according to this variable.
Finally, outcomes with oral anticoagulation were not evaluated in this study, which should be taken into account when interpreting our conclusions.
CONCLUSIONSIn this prospective registry carried out in patients with AF from a single health area, digoxin was not associated with increased all-cause mortality, survival free of admission due to any cause or survival free from admission due to cardiovascular causes, regardless of the presence or absence of underlying HF, even though patients with HF are at higher risk.
CONFLICTS OF INTERESTNone declared.
This work would not have been possible without the invaluable contributions of the participating physicians. The large number of participants does not permit the individual acknowledgments that we would like to make and which are well deserved.