To determine by confirmatory factor analysis whether a model of the metabolic syndrome including waist circumference-to-height ratio, as a measure of adiposity, has better goodness of fit than that based on waist circumference alone and, on the basis of the data of the best-fit model, to develop an index of global cardiometabolic risk in young adults.
MethodsCross-sectional observational study involving 683 university students aged 18 to 30years, in their first year at the University of Castilla-La Mancha in Spain, during the 2009-10 academic year. We compared the best fit of 2 models of the metabolic syndrome, both of which included the triglyceride-to-high-density lipoprotein cholesterol ratio, HOMA-IR index, and mean arterial blood pressure, but differed in that one of them used waist circumference, whereas the other used the waist circumference-to-height ratio. A metabolic syndrome index (MSI) was constructed and its association with aerobic capacity, daily physical activity and muscle strength was estimated.
ResultsThe single-factor model that included waist circumference was a better indicator of goodness of fit. The MSI was inversely associated with aerobic capacity and muscle strength.
ConclusionsThis study confirms that a single factor underlies the concept of metabolic syndrome; including the waist circumference-to-height ratio does not result in improvements over the model in which waist circumference alone is considered, and the development of a quantitative MSI may be useful for the quantification of cardiometabolic risk in clinical practice.
Keywords
The metabolic syndrome is a group of cardiometabolic disorders that is considered to be a predictor of cardiovascular disease, type 2 diabetes mellitus, and overall mortality.1, 2, 3 The definition of the metabolic syndrome is a subject of controversy, although all the currently accepted definitions include insulin resistance or glucose intolerance, hypertension, dyslipidemia, and central obesity.4
Exploratory factor analysis techniques can be used to analyze the interrelationship among a set of variables determined in a group of individuals and to establish a small group of latent variables that we refer to as factors.5 A number of studies have examined the relationship between the different phenotypic factors or traits that constitute the metabolic syndrome using factor analysis techniques, mainly principal component analysis. Most of the analyses included several measures of adiposity, arterial blood pressure, and fasting plasma concentrations of triglycerides, high-density lipoprotein cholesterol (HDLC), glucose and insulin.6, 7, 8 To date, however, there is no consensus as to the number of factors underlying the concept (or construct) of the metabolic syndrome. This is possibly due to the very nature of exploratory factor analysis, which is strongly conditioned by the method by which the factors are extracted, the type of rotation employed, and the minimum factor loading selected to assign a variable to a factor.
Confirmatory factor analysis (CFA) is a multivariate statistical method used to determine whether there are one or several latent causative factors underlying a clinical concept9 – in our case, the metabolic syndrome. Three studies affirm that a 4-factor model used in Europeans, Afro-Americans, and Hispanics explains the relationship between the variables of the metabolic cluster.10, 11, 12 Pladevall et al. identify a single-factor model in the metabolic syndrome that includes waist circumference, triglyceride-to-HDLc ratio, homeostatic model assessment of insulin resistance (HOMA-IR), and mean arterial pressure (MAP).
It has been pointed out that waist circumference-to-height ratio is the adiposity parameter most closely associated with the metabolic syndrome14 and has the greatest discriminatory capacity for hypertension, diabetes mellitus, and dyslipidemia both in men and women.15 Moreover, although the model proposed by Pladevall et al. has demonstrated good fit, it has not been determined whether a better fit is obtained in that model if waist circumference is substituted for the waist circumference-to-height ratio.
This report has the following objectives: a) to verify by means of CFA whether there is a a single factor underlying the concept of the metabolic syndrome; b) to compare the goodness of fit of a model of the metabolic syndrome that includes waist circumference as a measure of adiposity with another that includes the waist circumference-to-height ratio; c) on the basis of the best fit model, to develop an index of global cardiometabolic risk, as has been established for Spanish children16; and d) to estimate the relationship between this index and aerobic capacity, muscle strength, and physical activity.
Methods Subjects and Experimental DesignWe describe a cross-sectional observational study in which all the first-year university students on the Cuenca Campus of the Universidad de Castilla-La Mancha in Spain were invited to participate. Of the 770 students invited, 683 (88.7%) accepted.
The study protocol was approved by the Clinical Research Ethics Committee of Hospital Virgen de la Luz in Cuenca and, once they had been informed orally and in writing, all the subjects were asked for their signed consent as a condition to participate in the study.
Anthropometric VariablesDuring the 2009-2010 academic year, in addition to sociodemographic variables (age, sex, type of studies undertaken, etc.), for all subjects we collected the following data:
- Body weight: the mean of 2 measurements using an approved, easily calibrated Seca-770 scale, with the subject barefoot and wearing light clothing.
- Height: the mean of 2 measurements using a Seca-222 wall-mounted stadiometer, with the subject barefoot and standing erect, with his or her sagittal midline at the midline of the stadiometer.
- Body mass index: calculated as weight (in kg) / square of height (in m).
- Waist circumference: the mean of 2 measurements of the waist using a flexible tape measure (midway between the last rib and the iliac crest).
- Systolic and diastolic arterial blood pressure: the mean of 2 measurements separated by an interval of 5min, following a rest period of at least 5min prior to the first determination. The subject was seated, in a calm, quiet atmosphere. Arterial blood pressure was obtained by an automated procedure using the OMRON M5-I monitor.
The anthropometric measurements and arterial blood pressure readings were taken by nurses who had attended a series of training sessions.
Lipid and Metabolic VariablesBlood samples were collected via cubital vein puncture under standard17 conditions between 8:15 and 9:00 AM after at least 12h of fasting. The samples were processed in a COBAS C711 system from Roche Diagnostics, and the following biochemical parameters were determined: total cholesterol (CHOD-PAP enzymatic method), triglycerides (GPO-PAP enzymatic method), glucose (hexokinase method) and direct HDLC plus (second generation method without deproteinization). The blood insulin concentration was determined by 1-step chemiluminescent microparticle immunoassay and processing on a platform composed of 2 ARCHITECT i2000SR systems from Abbott Laboratories. Insulin sensitivity was determined on the basis of the HOMA-IR index18 using the formula: baseline glucose (mmol/L)×baseline plasma insulin (mU/mL) / 22.5.
Physical Activity and Fitness VariablesGiven that an association has been reported between physical activity, muscle strength, and aerobic capacity, independent of the incidence of the metabolic syndrome,19, 20 for the purpose of examining the relationship between the cardiometabolic risk index and these variables, we determined the following variables:
Aerobic CapacityMaximum oxygen consumption (VO2max) was determined by a submaximal exercise test on a cycle ergometer (Ergoline Variobike 550; Ergometrix, Barcelona, Spain) according to a protocol established by the FitMate device.21 The oxygen concentration was measured directly using the FitMate Pro gas analyzer (COSMED, Rome, Italy).
Muscle StrengthThis aspect was evaluated by means of 2 tests: standing long jump with feet shoulder width apart and handgrip dynamometry using a Takei TKK 5101 digital dynamometer (range, 5-100kg; accuracy, 0.1kg). These tests are validated and standardized and are part of the EUROFIT battery.22 We calculated a synthetic muscle strength index composed of the sum of the standardized z scores for the dynamometric test-to-weight ratio and the long jump test.
Physical ActivityA subsample of 273 subjects wore on their right hips, for 7 consecutive days, a MTI/CSA 7164 accelerometer (Actigraph, Shalimar, Florida, United States) programmed to record minute-by-minute movement. The intensity of weekly physical activity was evaluated as the average counts/min for the duration of the activity.
Statistical AnalysisWe tested two models of clustering of metabolic syndrome variables under a factor: that proposed by Pladevall et al.,13 which includes waist circumference, the triglyceride-to-HDLC ratio, HOMA-IR, and MAP (diastolic arterial pressure+1/3 [systolic arterial pressure – diastolic arterial pressure]), and another model in which the waist circumference variable is replaced by the waist circumference-to-height ratio.
The HOMA-IR index and the triglyceride-to-HDLC ratio were logarithmically transformed to fit them to a normal distribution, and all the variables of the model were standardized (mean=0 and standard deviation=1) according to age and sex. The factor weights (λ) corresponding to each of the variables measured indicate the strength of the association between the variables and the concept of the metabolic syndrome. We used a cut-off point of ±0.3 as the minimum level of practical significance of a λ coefficient.
The estimates of the parameters were obtained using maximum likelihood methods.
The fit of the models was analyzed in several ways. The χ2 test was utilized to assess the fit of the hypothetical models to the sample data. As the size of the sample was relatively large (n=683), goodness of fit was analyzed using the criteria of Hu et al.,23 which include the comparative fit index (CFI) and the standardized root mean square residual (SRMR). A model was considered to have good fit if the CFI value was greater than 0.96 and the SRMR value was less than 0.08.
Multiple group analysis was carried out to examine sex-related differences. We determined whether the differences in the factor weights were statistically significant using the χ2 difference test.
We calculated a new metabolic syndrome index (MSI) comprised of the sum of the standardized scores for the 4 variables that make up the model, multiplied by their factor weights. A web application has been developed for the automated calculation of the MSI on the basis of individual gross values (available at: http://www.cess.uclm.es/sm). The means of the MSI according to sex were compared using Student's t test.
Receiver operating characteristic (ROC) curves were generated to obtain the optimal cut-off point for the MSI, taking into account the Youden index24 for the prediction of risk of the metabolic syndrome in adult university students. As diagnostic criteria, we used that of the International Diabetes Federation,25 which, in addition to the presence of central obesity (waist circumference greater or equal to 94cm in men and 80cm in women), requires the presence of at least two of the following factors: triglycerides ≥150mg/dL, HDLC<40mg/dL in men and <50mg/dL in women, glucose ≥100mg/dL and elevated arterial blood pressure (systolic ≥130mmHg and/or diastolic ≥85mmHg).
Using covariance analysis adjusted for age and sex, we evaluated the differences in the average MSI according to categories of aerobic capacity, physical activity (counts/min) and a synthetic muscle strength index. These variables were recoded on the basis of the quartiles in 3 groups (Q1, Q2-Q3, and Q4), and linear trend analysis was performed using polynomial contrast. The P value of the post hoc contrast hypothesis was determined with the Bonferroni correction for multiple comparisons. The criteria for statistical significance of 2-sided testing was a P value less than or equal to .05. All the statistical analyses were performed with the SPSS 17.0 software package, except for the CFA, which was carried out with the AMOS 17.0 software package (SPSS Inc).26
ResultsThe final sample included 683 first-year university students, with ages between 18 and 30years (mean, 20.19±4.36years). Of these, 505 (73.9%) were women. The prevalence of the metabolic syndrome, according to the criteria of the International Diabetes Federation, was 2.2%.
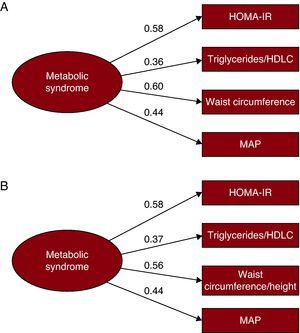
Figure 1 shows both models of a factor proposed to analyze the factor structure of the metabolic syndrome. Both the fit of the model proposed by Pladevall et al.13 (Figure 1A) (χ2=2.27; df=2; P=.321; CFI=0.99; SRMR=0.016) and that of the same model substituting waist circumference for the waist circumference-to-height ratio (Figure 1B) (χ2=2.76; df=2; P=.251; CFI=0.99; SRMR=0.018) were quite good. However, for the latter, the factor weight for the waist-to-height ratio variable (λ=0.56), was somewhat lower than that of the waist circumference variable of the first model (λ=0.6). For this reason, and since the first model was more parsimonious, we decided that it was more suitable for our purpose.
Figure 1. Factor weights and goodness of fit indices for 2 models of the structure of a factor of the metabolic syndrome. A: model proposed by Pladevall et al. 13 B: model proposed by Pladevall et al with the waist circumference-to-height ratio. HDLC, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; MAP, mean arterial pressure.
Subsequently, we tested the validity of the model of a factor that includes waist circumference, HOMA-IR, triglyceride-to-HDLC and MAP, checking for sex-related differences (Figure 2), and found that the estimate of the factor weights of the 4 metabolic syndrome measurements were similar in both men and women (χ2 differences=3.22; df=3; P=.359). The MSI values ranged between –3.24 and 4.92; the median was –0.02 and the 85th, 90th and 95th percentiles were 1.32, 1.6 and 2.08, respectively. According to sex, we found no significant differences between the mean MSI in men (-0.006±1.27) and women (0.01±1.25) (P=.895).
Figure 2. Factor weights and goodness of fit indices for 2 models of the structure of a factor of the metabolic syndrome according to sex. HDLC, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; MAP, mean arterial pressure.
Taking as standard diagnostic criteria those of the International Diabetes Federation, the MSI cut-off point that had the greatest area under the ROC curve was 1.48 (area=0.95; 95% confidence interval [CI], 0.91-0.99; sensitivity, 92%; specificity, 90%). This same cut-off point was that having the highest value in the Youden index (index=0.876; 95% CI, 0.66-0.92). The prevalence of the metabolic syndrome according to the cut-off point established for the MSI was 12%.
Figure 3 shows the differences in the mean MSI between the quartiles for aerobic capacity and the muscle strength index, according to sex and adjusting for age. The mean MSI became lower as the aerobic capacity increased, both in men (P=.001) and women (P=.009) and as the muscle strength index increased, again, in men (P=.001) and women (P=.001). There were no significant differences between the quartiles for total daily physical activity.
Figure 3. Difference in the average metabolic syndrome index in each of the categories of aerobic capacity and muscle strength according to sex. Q1: first quartile; Q2-Q3: second-third quartiles; Q4: fourth quartile; VO2max: maximal oxygen consumption (aerobic capacity). The muscle strength index was calculated as the sum of the standardized z scores for the dynamometry-to-weight ratio and the long jump test. The error bars represent the standard error of the mean.
DiscussionOur data confirm by means of CFA that, in young adults, there is a single factor behind the concept of the metabolic syndrome. Moreover, in our population, the model that includes waist circumference as a measure of adiposity is associated with better indicators of goodness of fit than that including the waist circumference-to-height ratio. Finally, we have developed an index to quantify the risk of the metabolic syndrome in which the average values improve as the aerobic capacity and muscle strength increase.
A number of studies have employed CFA to evaluate models of the metabolic syndrome in children,16 adolescents,27 and adults.10, 11, 12 There is no consensus in the literature as to the number of factors that comprise the concept of the metabolic syndrome. Pladevall et al.13 demonstrated the goodness of fit of a single-factor model that included only 1 variable as a representative measure of each of the 4 components that are included in the different definitions of the metabolic syndrome. This model involving a single factor, but having different variables, has also been tested in children16 and adolescents,27 and our study confirms that the model proposed by Pladevall et al. also shows a satisfactory fit in young adults in Castilla-La Mancha, Spain.
The waist circumference-to-height ratio has been reported to be more strongly associated with cardiovascular and metabolic risk than other anthropometric indices, both in adults28, 29 and children.30, 31 Thus, it seems logical to think that a conceptual model of the metabolic syndrome that includes the waist circumference-to-height ratio as a measure of adiposity should provide better fit than one in which this variable is replaced by waist circumference. However, in our population, both the factor weights and the indicators of goodness of fit of the model are somewhat better when we use waist circumference. This circumstance could be due to the fact that the variables that represent each component of our model are adjusted for age and sex and, thus, could indirectly be adjusted for height due to its close relationship with those variables.
In clinical practice, it is very useful to establish two diagnostic categories; however, given the relatively low prevalence in young adults, it is difficult to find enough power to establish associations between this syndrome and a number of lifestyle-related factors. For this reason, numerous studies aimed at relating cardiometabolic risk to different lifestyle-related factors have utilized summative indices on the basis of variables representing each component of the metabolic syndrome.32, 33, 34, 35 The European Association for the Study of Diabetes and the American Diabetes Association have even recommended the development of studies that justify the use of a scoring system based on continuous variables to measure the risk of the metabolic syndrome,36 similar to that employed in the Framingham37 score to quantify the risk of cardiovascular events.
A group of experts from the World Health Organization recently pointed out that the dichotomous nature of the diagnosis of the metabolic syndrome was a limitation to the clinical utility of its diagnosis.38 Given the innovation that the quantification of the measurement of a syndrome defined by quantitative variables represents for the future, perhaps the clinical contribution of the MSI should be interpreted in an index that considers these variables in their essence. In practice, this constitutes an advantage to the extent that an individual with none of the risk factors that comprise the syndrome, who therefore does not reach the established cut-off points, could present an MSI above the cut-off point for this variable; for the same reason, multifactorial interventions (changes in lifestyle, multiple drug therapy) that are not effective against a dichotomously defined metabolic syndrome could be beneficial in a metabolic syndrome defined using a quantitative scoring system.
Both aerobic capacity and muscle strength have been inversely associated with cardiometabolic risk.32, 33, 34, 39 However, the results of the studies dealing with the relationship between physical activity and cardiometabolic risk are contradictory.33, 35 The values of the MSI that we developed on the basis of factor analysis data improve as the aerobic capacity and muscle strength increase. The absence of an association between physical activity and the MSI could reflect agreement with studies with similar results33, 35 or could be due to the fact that cardiometabolic risk is modified only at certain levels of intensity of physical activity.40
Our results should be interpreted with caution, since they correspond to a cross-sectional study and, thus, do not establish a temporal relationship among the components of the metabolic syndrome. Another possible limitation concerns the fact that the MSI has been calculated on the basis of a specific sample of university students under the age of 30years and, unless the sociodemographic characteristics and the cardiovascular risk profile are similar to those of our study population, it cannot be compared with the other cardiometabolic indices utilized to date.
Perhaps the greatest strength of the MSI that we propose is the fact that, in contrast to other indices,33, 35, 41 ours considers the factor weights associated with each of the variables that comprise the CFA model. Moreover, the development of a computer application to calculate the metabolic risk in young adults may help it to become more widely used in clinical practice.
ConclusionsOur study corroborates the affirmation that a single factor underlies the concept of the metabolic syndrome and the fact that inclusion of the waist circumference-to-height ratio does not improve the goodness of fit of the model and makes it less parsimonious. Moreover, this report provides a new index for the quantification of cardiometabolic risk and the development of a web site for its calculation.
FundingThis study has been funded by the Fundación para la Investigación Sanitaria (Health Research Foundation) in Castilla-La Mancha, Spain (FISCAM; ref. AN/2008/31) and the Ministry of Health and Consumer Affairs, Instituto de Salud Carlos III, Red de Investigación en Actividades Preventivas y de Promoción de Salud (Network for Research in Preventive Activities and Health Promotion) (ref. RD06/0018/0038).
Conflicts of interestNone declared.
Acknowledgments
The authors thank all the participants in the Estudio de Cuenca Adultos.
Researchers Participating in The Estudio de Cuenca Adultos
Principal investigator: V. Martínez Vizcaíno. Universidad Castilla-La Mancha.
Cuenca: M. Solera Martínez, M. Sánchez López, B. Notario Pacheco, P. Moya Martínez, N. Arias Palencia, R.M. Fuentes Chacón, M.V. Ungría Cañete, R. Olmo Gascón. Universidad de Castilla-La Mancha.
P. Franquelo Morales, P. Franquelo Gutiérrez, S. López Martínez. Hospital Virgen de la Luz. SESCAM.
Salamanca: L. García Ortíz, M.A. Gómez Marcos. Centros de Salud (Health Centers) La Alamedilla and Garrido Sur.
Madrid: F. Rodríguez Artalejo. Universidad Autónoma de Madrid.
Bilbao: Gonzalo Grandes. Unidad de Investigación de Atención Primaria (Primary Care Research Unit). Osakidetza.
Stockholm: J.R. Ruiz, F.B. Ortega. Instituto Karolinska.
Received 18 August 2010
Accepted 24 November 2010
Corresponding author: Centro de Estudios Sociosanitarios, Universidad de Castilla-La Mancha, Edificio Melchor Cano, Santa Teresa Jornet s/n, 16071 Cuenca, Spain. Montserrat.Solera@uclm.es