Detection of acute allograft rejection in heart transplant recipients by noninvasive methods is a challenge in the management of these patients. In this study, the usefulness of a new highly sensitive method for the measurement of troponin T is evaluated.
MethodsWe designed a case-crossover study, in which each patient served as his or her own control, by selecting samples from treated acute rejection episodes (29 cases) and samples obtained immediately before and/or after rejection (38 controls). The highly sensitive troponin T was measured by a new pre-commercial test (Elecsys Troponin T HS).
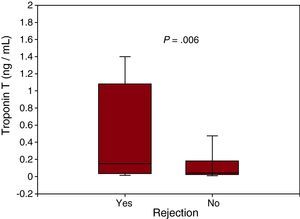
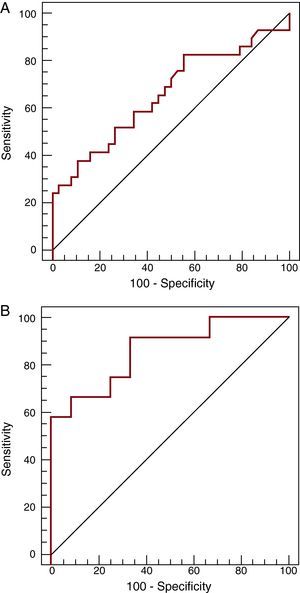
ResultsIn all samples, highly sensitive troponin was detectable, with a median of 0.068 ng/mL (IQR, 0.030-0.300 ng/mL). The levels correlated with right atrial pressure (r=0.37; P=.002), N-terminal pro-brain natriuretic peptide concentration (r=0.67; P<.001), and time since transplantation (r=–0.81; P<.001). The highly sensitive troponin concentrations were higher in patients with rejection (0.155 ng/mL vs 0.047 ng/mL; P=.006). In the receiver operating characteristic analysis, the area under the curve was 0.67 (95% confidence interval, 0.53-0.77) and the best cutoff was 0.035 ng/mL, which was associated with rejection (odds ratio=3.7; 95% confidence interval, 1.2-11.9; P=.02). By restricting the analysis to the first 2 months, the area under the curve increased to 0.86 (95% confidence interval 0.66-0.97), with an optimal cutoff of 1.10 ng/mL (S=58% [28%-85%]; E=100% [74%-100%]).
ConclusionsTroponin T was detectable in all samples when a new highly sensitive assay was used, and at higher concentrations in the presence of acute rejection; however, the usefulness of this test in patient management is limited to support for clinical or histological suspicion of rejection, especially in the early post-transplant period.
Keywords
Despite the current advances in immunosuppressive therapy, 20% to 30% of patients undergoing heart transplantation require an increased immunosuppression level for acute cellular or humoral rejection.1, 2 The associated mortality is 6% in the first month and reaches 12% at the end of the first year.3 Currently, endomyocardial biopsy (EMB) is the standard tool for the diagnosis of acute rejection, despite its invasive nature and low sensitivity due to considerable variability in sampling and in intraobserver and interobserver interpretation.
Determination of cardiac troponins in blood is a standard method for prompt detection of ischemic injury in acute coronary syndromes. Acute rejection is also associated with cardiomyocyte necrosis and, therefore, with release of cardiac troponins.4, 5 Nonetheless, the low sensitivity of conventional techniques for troponin determination limits the clinical applicability of this test for heart transplant recipients, in whom the initial troponin release is of very low magnitude.6 In recent years, highly sensitive methods with significantly lower limits of detection have been developed for troponin determination.7 Thus, these tests might be feasible for less invasive clinical monitoring of acute allograft rejection.
The aim of this study was to evaluate a new, highly sensitive method for troponin T (hsTnT) detection in the diagnosis of acute rejection in heart transplant recipients.
METHODS Population and Study DesignA case-crossover study was designed in which each patient served as his or her own control, by selecting samples obtained during a rejection episode (cases) and samples taken immediately before or after the episode (controls). Between 2000 and 2008, 72 heart transplants were performed, in which EMB was carried out as part of the regular monitoring protocol or for clinically suspected rejection. Blood samples were drawn immediately before each EMB, as required in the Transplant Immunology protocol of the research group network (File G03/114; Instituto de Salud Carlos III), and serum was obtained, processed, and frozen at -80°C. Within this total population, 29 (40%) patients (mean age, 53±13 years; 75% males) presented a first acute rejection episode during the first year, as defined by treatment with an intravenous bolus of methylprednisolone at a dose of ≥250mg based on clinical or histological criteria of rejection. We selected samples obtained during the rejection episode (n=29 cases, rejection group) and those obtained in the biopsy performed immediately before (n=17) and/or after (n=21) rejection (n=38 controls, no rejection group). For each biopsy, the degree of histological rejection was classified in accordance with the criteria of the International Society of Heart and Lung Transplantation.8 The clinical and laboratory characteristics at the time of biopsy were recorded prospectively on the patient's medical chart, in keeping with the regular clinical practice for this population, and the data were later collected for the study analysis.
Laboratory MeasurementsSamples underwent a single thawing cycle before troponin T determination using a highly sensitive electrochemiluminescence immunoassay (Elecsys Troponin T hs) on an Elecsys 2010 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The test has an analytic range of 0.003 to 10ng/mL, a lower detection limit of 0.003 ng/mL, and a value of 0.013 ng/mL for the 99th percentile of the normal population (coefficient of variation for this value, 9%). This commercial test was validated recently, and meets the consensus requirements and recommendations for use in the diagnosis of myocardial necrosis.9 Concentrations of N-terminal pro-brain natriuretic peptide (NT-proBNP) were also measured using the above-mentioned Elecsys 2010 system; total imprecision of the technique was <3%.
Statistical AnalysisDifferences between the rejection and nonrejection groups were analyzed with the Student t test for variables with a normal distribution or the nonparametric Mann-Whitney U test for those with nonnormal distribution. The chi-square test was used to compare qualitative variables. To study possible influences on the hsTnT values obtained, linear multiple regression analysis was carried out, including in the model variables that showed a significant correlation. The diagnostic utility of hsTnT for predicting acute cellular rejection was evaluated with a receiver operating characteristic (ROC) analysis, calculating the area under the curve and confidence interval (CI) using the DeLong method.10 The best cut-off for the diagnosis was the one in which the highest product was obtained by multiplying the specificity by the sensitivity. The association of risk with rejection was determined with logistic regression analysis, adjusted by other significant variables. P values <.05 were considered statistically significant. Statistical calculations were performed with MedCalc 11.3.0 (MedCalc Software, Mariakerke, Belgium) for the ROC analysis, and with PASW 18.0 (SPSS Inc., Chicago, Illinois) for the other analyses.
RESULTSThe clinical and biochemical characteristics according to the presence or absence of rejection are shown in Table 1. In the overall population, troponin T was detectable in all samples (100%) at a median of 0.068 (0.030-0.300) ng/mL, and significantly higher concentrations were found in patients with rejection (Figure 1). When the population was stratified into tertiles of troponin T concentration, there was also an association with a higher prevalence of rejection (P=.02): 23% (<0.035 ng/mL), 48% (0.035-0.176 ng/mL), and 59% (>0.176 ng/mL). Clinical variables correlating with troponin T values are shown in Table 2. Following adjustment with multiple linear regression, the time since transplantation, right atrial pressure, and NT-proBNP concentration were found to be the main determinants. There was no correlation between troponin T concentration and histological grade of rejection (P>.5).
Table 1. Baseline Characteristics of Endomyocardial Biopsies With and Without Rejection.
| Rejection (n=29) | No rejection (n=38) | P | |
| Time since transplant, months | 2.6 [1.1-6.7] | 3.6 [1.5-8.8] | .152 |
| Cyclosporin, ng/mL a | 270±221 | 237±102 | .516 |
| Tacrolimus, ng/mL b | 10.9±3.7 | 14.3±9.5 | .418 |
| Mycophenolate acid, μg/mL | 2.7±2.2 | 2.6±1.5 | .734 |
| LVEF, % | 60 [56-64.5] | 60 [59-62.7] | .396 |
| Right atrial pressure, mmHg | 10 [5-15] | 8 [4-11] | .559 |
| RV systolic pressure, mmHg | 37 [29-43] | 36 [30-41] | .556 |
| Creatinine, mg/dL | 1.3±0.6 | 1.3±0.5 | .8 |
| Ureic nitrogen, mg/dL | 62±37 | 57±33 | .605 |
| Uric acid, mg/dL | 7.1±2.6 | 6.6±2 | .453 |
| Albumin, g/dL | 3.6±0.8 | 3.86±0.42 | .152 |
| C reactive protein, mg/dL | 1.1 [0.3-3.7] | 0.5 [0.1-1.4] | .065 |
| Hemoglobin, g/dL | 11±1.5 | 11.8±1.5 | .038 |
| NT-proBNP, ng/L | 3684 [916-12005] | 1587 [778-4864] | .047 |
| Troponin T, ng/mL | 0.155 [0.040-1.080] | 0.047 [0.026-0.187] | .006 |
Values are expressed as the mean±standard deviation or median [interquartile range].
LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; RV, right ventricle.
a n=18 for cyclosporine.
b n=11 for tacrolimus.
Figure 1. Troponin T concentration in endomyocardial biopsies with and without rejection.
Table 2. Variables Related to Troponin T Concentration in the Linear Regression Analysis.
| Univariate | Multivariate | |||
| r | P | β | P | |
| Time since transplant | –0.810 | <.001 | –0.600 | <.001 |
| Right atrial pressure | 0.370 | .002 | 0.241 | .021 |
| NT-proBNP | 0.666 | <.001 | 0.220 | .046 |
| C-reactive protein | 0.384 | .002 | –0.042 | .649 |
| Hemoglobin | –0.356 | .003 | –0.131 | .136 |
NT-proBNP, N-terminal pro-brain natriuretic peptide.
The ROC analysis yielded an area under the curve of 0.67 (95% CI, 0.53-0.77) (Figure 2A) for the presence of rejection. The optimal cut-off point was 0.035 ng/mL, which had a sensitivity of 83% (95% CI, 64%-94%), specificity of 45% (95% CI, 29%-62%), positive predictive value of 56%, and negative predictive value of 77%. In the multivariate analysis, troponin T was associated with a higher risk of rejection (P=.02; odds ratio [OR] 3.7; 95% CI, 1.2-11.9) after adjusting for time since transplantation, right atrial pressure, and NT-proBNP concentration. When ROC analysis was restricted to the first 2 months following transplantation (12 samples with rejection and 12 controls with no rejection), the area under the curve increased to 0.86 (95% CI, 0.66-0.97) (Figure 2B), and the optimal cut-off point rose to 1.10 ng/mL, with a sensitivity of 58% (95% CI, 28%-85%), specificity of 100% (95% CI, 74%-100%), positive predictive value of 100%, and negative predictive value of 66%.
Figure 2. ROC curves in highly sensitive troponin T determination for detecting rejection in the total population studied (A, n=67) and in the first 2 months post-transplantation (B, n=24).
DISCUSSIONBecause of the limitations of EMB, several noninvasive alternatives have been investigated for the detection of acute cardiac rejection.5, 8, 11, 12, 13, 14 Among them, determination of cardiac troponins with conventional techniques has also been assessed, with conflicting findings. In some biopsy series, troponin concentrations have shown a correlation with the histological grade of rejection,5, 6 whereas in others, this association was not found.11, 12, 15, 16 Nonetheless, all these studies have reported poor sensitivity and a low positive predictive value for these determinations.
The present study shows that with the use of a highly sensitive assay, troponin T was detectable in all samples of patients with and without rejection. When conventional techniques are used, 54% of patients present persistently undetectable concentrations. This finding concurs with the high sensitivity these tests have shown in patients with suspected coronary disease.17, 18 Moreover, the concentrations were significantly higher in cases of rejection requiring intravenous steroid treatment based on clinical or biopsy findings. The area under the curve was relatively low, however, probably because of the strong influence of the time since transplantation (r=–0.8).
Sensitivity and negative predictive values would have to be high to avoid biopsy when monitoring rejection in heart transplantation, and in this regard the values obtained for hsTnT were relatively low. Therefore, hsTnT monitoring would not avert the need for EMB, although the positive association with rejection found in this study indicates that it could be of use to support suspected rejection based on clinical or biopsy findings. In this sense, diagnostic capacity was higher in the early period, in which elevated troponin values were associated with a high positive predictive value (100%); thus, in cases in which there is no post-transplant decrease or when the concentration is greater than 1.10 ng/mL, hsTnT determination could help to support a suspicion of acute rejection during the first few months following transplantation.
One point of interest in our population was that no association was found with histological grade, as has been described in related studies investigating conventional tests.11, 12, 15, 16 This could indicate that histological study of endomyocardial biopsy material is not a perfect tool to use as the only reference. In fact, the molecular changes occurring in rejection correlate better with the clinical findings than with the histological lesions, as was recently demonstrated by Mengel et al.19 In our analysis, the correlation of hsTnT with filling pressures and NT-proBNP also indicates a clinical correlation with hemodynamic variables that are affected by the presence of rejection with clinical repercussions.20, 21
This study is limited by its observational character and the small number of samples obtained in the different time periods. However, this is due to the fact that heart transplantation is not a common procedure and rejection episodes are unpredictable events in these patients. The main strength of the study is that it evaluates for the first time troponin test with a highly sensitive technique, and can serve to increase the knowledge in this area and be of help in the design of new studies focused on the search for noninvasive markers of rejection. In any case, further studies with a prospective design are needed to better define the role of hsTnT with or without other biological markers for monitoring rejection following heart transplantation.
CONCLUSIONSThe findings of this study show that with the use of a highly sensitive test, troponin T is measurable in all patients following transplantation, and concentrations are higher in patients with acute rejection. Furthermore, persistently elevated hsTnT values in the early postoperative period are associated with a higher risk of rejection, although their use for later monitoring should be individualized and always considered a support parameter for clinically or histologically suspected rejection.
CONFLICTS OF INTERESTDr. Domingo Pascual-Figal has received research grants from Roche Diagnostics.
Acknowledgments
The reagents used for hsTnT determination were kindly donated by Roche Diagnostics.
Received 29 March 2011
Accepted 19 June 2011
Corresponding author: Servicio de Cardiología, Hospital Universitario Virgen de la Arrixaca, Ctra. Madrid-Cartagena s/n, 30120 Murcia, Spain. dapascual@servicam.com