The prognostic benefit of statins in patients with heart failure is a topic of controversy. Under the hypothesis that statins may provide greater benefit in a subgroup of patients with heightened inflammatory activity, we sought to explore whether statins are associated with a decreased risk of long-term mortality in patients with acute heart failure based on elevated levels of carbohydrate antigen 125, a biomarker related to systemic congestion and proinflammatory status.
MethodsWe analysed 1222 consecutive patients admitted with acute heart failure in a single teaching center during a median follow-up of 20 months. Carbohydrate antigen 125 was measured during index hospitalization and dichotomized according to the established reference cut-off (>35 U/mL).
ResultsIncreased levels of carbohydrate antigen 125 (>35 U/mL) were observed in 793 (64.9%) and prescription of statins registered in 455 (37.2%) patients. In patients with carbohydrate antigen 125 >35 U/mL, mortality was lower in statin-treated patients (1.89 vs 2.80 per 10 patient-years of follow-up, P<.001). Conversely, in those with carbohydrate antigen 125 in normal range, mortality did not differ (1.76 vs 1.63 per 10 patient-years of follow-up, P=.862). After covariate adjustment, this differential effect persisted (P for interaction=.024) and statin use was associated with a significant mortality reduction in patients with elevated values of carbohydrate antigen 125 (hazard ratio=0.65, 95% confidence interval: 0.51-0.82; P<.001), but not in those with values equal to or below 35 U/mL (hazard ratio=1.02, 95% confidence interval: 0.74-1.41; P=.907).
ConclusionsElevation of carbohydrate antigen 125 (>35 U/mL) identified a subset of patients with acute heart failure who could benefit from statin treatment in regard to total mortality.
Keywords
Inflammation is a key pathogenic process associated with the progression of heart failure (HF).1 The pleiotropic anti-inflammatory properties of statins appear to be an attractive feature for targeting the inflammatory component in patients with advanced HF.2 In contrast to large-scale observational studies that have shown a reduction in clinical outcomes in patients with HF and treated with statins,3, 4, 5 two recent randomized controlled trials of statins in HF failed to demonstrate any survival benefit.6, 7 Nevertheless, a post hoc analysis of the CORONA trial showed decreased mortality with rosuvastatin in patients exhibiting high inflammatory activity as measured by serum C-reactive protein (CRP) (>2mg/dL).8.
Accumulated evidence has pointed to carbohydrate antigen 125 (CA125) as a reliable marker for congestion and inflammation in patients with acute heart failure (AHF)9, 10, 11, 12 and as being independently associated to all-cause and cardiovascular mortality.11, 12 The fact that serum levels of CA125 have shown to be very reliable over time12, 13 has led to postulating this biomarker as an ideal candidate for measuring the degree of inflammation in AHF. Thus, and assuming that patients with AHF and high CA125 levels (>35 U/mL) represent a subset of patients with elevated inflammatory activity, we sought to evaluate whether statin treatment following an episode of AHF has differential prognostic effect in terms of total and cardiovascular mortality according to CA125 categories.
METHODS Study Group and ProtocolWe prospectively studied a cohort of 1222 patients consecutively admitted to the cardiology department of a third level center from January 1, 2004 to November 1, 2009 with the diagnosis of AHF as defined by current guidelines.14, 15, 16 By design, patients who died before CA125 measurement were excluded from this analysis (n=21). In addition, patients with a primary diagnosis of acute coronary syndrome (n=20), cancer (n=18), pneumonia (n=16), sepsis (n=8), severe hepatic disease (n=1), or end-stage renal disease undergoing dialysis treatment were also excluded (n=3).
Demographic information, medical history, vital signs, 12-lead electrocardiogram, laboratory data, and drug utilization were routinely determined on admission and throughout the hospital course, using pre-established registry questionnaires. All patients received intravenous treatment with furosemide for at least the first 48h after admission. Left ventricular ejection fraction (LVEF) was assessed through echocardiography (Agilent Sonos 5500-Phillips) during index hospitalization. Treatment with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, aldosterone antagonist, anticoagulants, diuretics, and other therapeutic strategies was individualized following established guidelines in force at the time of recruitment.14, 15, 16 The statin treatment decision was made at the discretion of the cardiologist in charge of the patient and was not influenced nor guided by CA125 values.
Patients were followed until death, and censored if lost to follow-up or by having undergone valve replacement or cardiac transplantation. All-cause mortality was selected as the main endpoint and cardiovascular mortality as a secondary one. The information regarding cause of death was extracted from the patient's clinical chart and adjudicated by an investigator blinded to patients’ treatment and CA125 values. Once identified, the cause of death was categorized following the classification used by the American Heart Association.17 Deaths were considered non-cardiovascular in origin if a specific non-cardiovascular cause was identified as the main trigger for the event. Otherwise, cardiovascular etiology was considered and included sudden death, progressive HF death, other cardiovascular causes, and unknown cause of death. Sudden death was defined as an event that occurred unexpectedly in an otherwise stable patient and progressive HF death as occurring in the setting of clinical progressive deterioration of HF symptoms. For the present study, deaths occurring outside the hospital were assumed to be cardiovascular in origin, whether information about the circumstances surrounding the death was provided by family members or by reviewing outpatient charts. This study conforms with the principles outlined in the Declaration of Helsinki, was approved by an institutional review committee, and patients gave informed consent.
Carbohydrate Antigen 125 MeasurementsCA125 was measured during the patient's hospitalization (72±12h after admission) using commercially available immunoassay kits (Elecsys CA125 II assay-Roche Diagnostics).
Statin TreatmentPatients were considered taking statins if they were prescribed at hospital discharge or, in cases of early death, only when treatment was initiated at least 24h before death. No specific guidelines were followed for initiation of statin treatment or selection of specific class or dosage. Based on low-density lipoprotein (LDL)18 and CRP reduction efficacy,19 the therapeutic equivalence between statins was characterized as low and medium-high doses. Low dose included atorvastatin ≤10mg, simvastatin ≤20mg, pravastatin ≤40mg, lovastatin ≤40mg or fluvastatin ≤80mg, and medium-high dose included atorvastin ≥20mg or simvastatin ≥40mg.
Statistical AnalysisContinuous variables were expressed as mean±1 standard deviation or median (interquartile range [IQR]) when appropriate. Discrete variables were presented as percentages. Mortality rates across statin therapy and CA125 categories (≤ or >35 U/mL) were depicted using the Kaplan-Meier method and their differences tested by the Peto-Peto Prentice test. The independent association between statin treatment and long-term mortality was assessed with Cox regression analysis. For the secondary analysis, cardiovascular death was independently modeled with Cox adapted for competing risk events.20 Candidate covariates for the initial multivariable model were chosen based on previous medical knowledge (see variables in Table 1), and independent of their P-value. A reduced, parsimonious, although highly predictive model was then derived by a backward elimination procedure using the “multivariable fractional polynomial” algorithm. The proportionality assumption for the hazard function over time was tested by means of the Schoenfeld residuals. The discriminative ability of the models was assessed using Harrell′s C-statistics and their calibration by the Gronnesby and Borgan test. Covariates that were included in the final models are specified in the respective figure and table legends. To provide clinical meaning to the Cox regression results, absolute measures for the association between statins and mortality were calculated. Adjusted absolute risk differences were estimated across CA125-binary status and at specific time points during the follow-up (3 and 6 months, 1, 3 and 5 years).21 Number needed to treat (NNT) and their 95% confidence interval (95%CI) were obtained by taking the reciprocal of the risk difference.
Table 1. Baseline Characteristics of the Population Stratified by Statin Therapy.
| All (n=1222) | Statins (n=455) | No statins (n=767) | P | |
| Demographic and medical history | ||||
| Age, years | 73±11 | 73±10 | 73±12 | .559 |
| Female, n (%) | 620 (50.7) | 211 (46.4) | 409 (53.3) | .019 |
| Previous admission for AHF, n (%) | 458 (37.5) | 170 (37.4) | 288 (37.5) | .948 |
| Hypertension, n (%) | 946 (77.4) | 393 (86.4) | 553 (72.1) | <.001 |
| Dyslipidemia, n (%) | 523 (42.8) | 342 (75.2) | 181 (23.6) | <.001 |
| Current smoker, n (%) | 128 (10.5) | 53 (11.6) | 75 (9.8) | .302 |
| Previous smoker, n (%) | 232 (19) | 109 (24) | 123 (16) | .001 |
| Ischemic heart disease, n (%) | 466 (38.1) | 261 (57.4) | 205 (26.7) | <.001 |
| Valvular heart disease, n (%) | 350 (28.6) | 97 (21.3) | 253 (33) | <.001 |
| ADHF, n (%) | 836 (68.4) | 293 (64.4) | 543 (70.8) | .020 |
| Acute pulmonary edema, n (%) | 265 (21.7) | 119 (26.1) | 146 (19) | .004 |
| Hypertensive AHF, n (%) | 99 (8.1) | 41 (9) | 58 (7.6) | .369 |
| NYHA class III/IV, n (%) a | 224 (18.3) | 79 (17.4) | 145 (18.9) | .501 |
| Previous HF, n (%) | 434 (35.5) | 165 (36.3) | 269 (35.1) | .674 |
| COPD, n (%) | 265 (21.7) | 108 (23.7) | 157 (20.5) | .180 |
| PAD, n (%) | 87 (7.1) | 43 (9.4) | 44 (5.7) | .015 |
| Stroke, n (%) | 126 (10.3) | 58 (12.7) | 68 (8.9) | .031 |
| Renal failure, n (%) | 205 (16.8) | 93 (20.4) | 112 (14.6) | .008 |
| Radiological pleural effusion, n (%) | 539 (44.1) | 185 (40.7) | 354 (46.1) | .061 |
| Peripheral edema, n (%) | 680 (55.6) | 238 (52.3) | 442 (57.6) | .070 |
| Previous use of diuretics, n (%) | 756 (61.9) | 288 (63.3) | 468 (61) | .428 |
| Previous use of beta-blockers, n (%) | 292 (23.9) | 144 (31.6) | 148 (19.3) | <.001 |
| Previous use of ACEI/ARB, n (%) | 554 (45.3) | 250 (54.9) | 304 (39.6) | <.001 |
| Previous use of statins, n (%) | 330 (27) | 260 (57.1) | 70 (9.1) | <.001 |
| Vital signs | ||||
| Heart rate, bpm | 101±29 | 99±28 | 102±30 | .018 |
| SBP, mmHg | 150±36 | 152±36 | 149±36 | .152 |
| DBP, mmHg | 83±20 | 83±21 | 82±20 | .291 |
| Electrocardiogram | ||||
| Atrial fibrillation, n (%) | 539 (44.1) | 153 (33.6) | 386 (50.3) | <.001 |
| QRS >120ms, n (%) | 358 (29.4) | 139 (30.5) | 219 (28.7) | .494 |
| Laboratory | ||||
| Haemoglobin, g/dL | 12.7±1.9 | 12.8±1.9 | 12.6±1.9 | .090 |
| Serum creatinine, mg/dL | 1.3±0.6 | 1.3±0.6 | 1.3±0.6 | .146 |
| Serum urea, mg/dL | 63±35 | 63±29 | 63±38 | .975 |
| Uric acid, mg/dL | 7.9±2.4 | 7.7±2.2 | 8±2.5 | .081 |
| Sodium, mEq/L | 139±5 | 139±4 | 139±5 | .459 |
| Troponin I, ng/mL | 0 [0-0.23] | 0.2 [0-0.33] | 0 [0-0.17] | <.001 |
| Troponin I >0.2 ng/mL, n (%) | 336 (27.5) | 150 (33) | 186 (24.2) | .001 |
| BNP, pg/mL | 145 [85-292] | 136 [72-284] | 151 [89-302] | .014 |
| Relative lymphocyte count, % | 17.9±9.9 | 18.9±9.9 | 17.3±9.9 | .008 |
| CA125, U/mL | 57 [25-129] | 53 [24-113] | 59 [26-141] | .021 |
| Total cholesterol, mg/dL | 170±44 | 177±47 | 166±41 | <.001 |
| Triglycerides, mg/dL | 120±57 | 135±65 | 110±49 | <.001 |
| LDL cholesterol, mg/dL | 104±35 | 107±40 | 102±32 | .019 |
| HDL cholesterol, mg/dL | 42±12 | 44±12 | 42±13 | .016 |
| Echocardiography | ||||
| LVEF, % | 51±15 | 49±15 | 52±15 | <.001 |
| LVEF ≤50%, n (%) | 560 (45.8) | 243 (53.4) | 317 (41.3) | <.001 |
| LAD, mm | 44±8 | 43±7 | 44±8 | .001 |
| LVDD, mm | 56±9 | 57±9 | 55±9 | <.001 |
| Medical treatment | ||||
| Beta-blockers, n (%) | 635 (52) | 258 (56.7) | 377 (49.1) | .011 |
| Diuretics, n (%) | 1198 (98) | 444 (97.6) | 754 (98.3) | .379 |
| Spironolactone, n (%) | 232 (19) | 97 (21.3) | 135 (17.6) | .109 |
| ACEI, n (%) | 505 (41.3) | 170 (37.4) | 335 (43.7) | .030 |
| ARB, n (%) | 361 (29.5) | 159 (34.9) | 202 (26.3) | .001 |
| Oral anticoagulants, n (%) | 493 (40.3) | 150 (33) | 343 (44.7) | <.001 |
| Nitrates, n (%) | 246 (20.1) | 134 (29.4) | 112 (14.6) | <.001 |
| Digoxin, n (%) | 313 (25.6) | 94 (20.7) | 219 (28.5) | .002 |
ACEI, angiotensin converting enzyme inhibitors; ADHF, acute decompensate heart failure; AHF, acute heart failure; ARB, angiotensin receptor blockers; BNP, brain natriuretic peptide; CA125, antigen carbohydrate 125; COPD, chronic pulmonary obstructive disease; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HF, heart failure; LAD, left atrial diameter; LDL, low-density lipoprotein; LVDD, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PAD, peripheral artery disease; SBP, systolic blood pressure.
Data are expressed as mean±standard deviation, n (%) or median [interquartile range].
a Last NYHA functional class measured under clinically stable conditions.
A 2-sided P-value of <.05 was considered to be statistically significant for all analyses. All analyses were performed using STATA 11.1 (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, Texas: StataCorp LP, United States).
RESULTSThe mean age in our sample was 73±11 years; 50.8% were female, 54% exhibited LVEF >50%, 64.9% had higher levels of CA125 (>35 U/mL) and 37.2% received statin therapy. Among patients that received statins, atorvastatin (61.5%), pravastatin (20.7%), and simvastatin (15.4%) were, in that order, the most frequent prescriptions. Among patients receiving statins, the median [IQR] dose for atorvastatin, pravastatin, and simvastatin were 20mg (20-40), 40mg (20-40) and 20mg (10-20), respectively. According to statin dose categorization, 210 (46.2%) and 245 (53.8%) patients were treated with low and medium-high dose, respectively. Clinical characteristics of the study population are shown in Table 1.
Clinical Predictors of Carbohydrate Antigen 125 ElevationPatients with CA125 values >35 U/mL displayed the worst baseline risk profile as evidenced by higher proportion of admission as acute decompensated heart failure, NYHA III/IV at stable phase of the disease, pleural effusion, peripheral edema, atrial fibrillation, and left ventricular systolic dysfunction. Likewise, higher means of uric acid, brain natriuretic peptide (BNP), and left atrial and ventricular dimensions, and lower means of systolic and diastolic blood pressure, serum hemoglobin, relative lymphocyte count, cholesterol (total, LDL and high-density lipoprotein [HDL]), and triglycerides were observed in this group of patients (supplementary material, annex 1). The most important variables that were independently associated with CA125 >35 U/mL were: a) presence of radiologic pleural effusion; b) BNP serum values, and c) peripheral edema, explaining 50.9%, 13.9% and 8% of the total R2, respectively.
Clinical Predictors of Statin PrescriptionTable 1 lists those variables significantly associated with statin prescription. The most important independent predictors (ranked in order of importance) were: a) previous treatment with statins (60% of total R2); b) history of dyslipidemia (12.8% of total R2), and c) history of myocardial infarction (7.5% of total R2). In this population, serum lipid levels contributed marginally to the odds of statin prescription (5.7%, 5.2%, and 2.6% of total R2 for HDL, LDL and triglycerides, respectively). It is notable that CA125, whether evaluated as a continuous or binary (CA125 >35 U/mL) variable, was not independently associated with statin prescription (odds ratio [OR]=0.99; 95%CI: 0.99-1.00, P=.524 and OR=1.29; 95%CI: 0.90-1.87, P=.168, respectively).
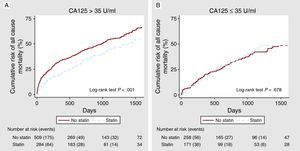
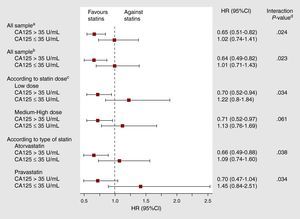
Statin Therapy and All-Cause Mortality Across Carbohydrate Antigen 125 StatusAt a median follow-up of 20 months (IQR=8-38), 542 (44.4%) deaths were ascertained. Of these, 418 were attributed as cardiovascular (77.1%) and 194 (35.8%) were due to HF progression. In univariate analysis, a differential prognostic effect between statin treatment and CA125 categories was observed (P for interaction=.019). In patients with CA125 >35 U/mL, mortality was lower in statin-treated patients (1.89 vs 2.80 per 10 patient-years of follow-up, respectively, P<.001), with differences observed since the first months of follow-up and reaching a maximum between the first and second year (Figure 1A). Conversely, in those with CA125 ≤35 U/mL, mortality rates did not differ by statin treatment (1.76 vs 1.63 per 10 patient-years of follow-up, P=.862) (Figure 1B). After a thorough multivariable adjustment, this interaction persisted (P for interaction=.024): treatment with statins was associated with a significant adjusted risk reduction in patients with elevated values of CA125 (HR=0.65, 95%CI: 0.51-0.82; P<.001), but not in those with values equal or below 35 U/mL (HR=1.02, 95%CI: 0.74-1.41; P=.907). This interaction was also present when CA125 was evaluated as continuous or dichotomized by its median value (supplementary material, annex 2). Moreover, several sensitivity analyses were performed with the aim to further support our findings. All of them confirmed the prognostic benefit of statins in patients with CA125 >35 U/mL: a) by forcing in the model the most important variables associated to statin prescription (previous treatment with statins, history of dyslipidemia, previous myocardial infarction, and raw lipid values); b) by testing statins dosages (low vs medium-high), and c) by testing, independently, the predictive value of the two most frequent statin classes prescribed (atorvastatin and pravastatin) (Figure 2).
Figure 1. Cumulative risk of mortality across statin therapy. A: In patients with carbohydrate antigen 125 >35 U/mL. B: In patients with carbohydrate antigen 125 ≤35 U/mL. CA125, carbohydrate antigen 125.
Figure 2. Total mortality adjusted hazard ratio (95% confidence interval) for the effect of statins among patients with carbohydrate antigen 125 >35 U/mL. CA125, carbohydrate antigen 125; 95%CI, 95% confidence interval; HR, hazard ratio. aModel 1: final multivariate Cox model adjusted by: age, gender, previous admission for acute heart failure (yes/no), admission as acute decompensate heart failure (yes/no), last New York Heart Association functinal class at stable phase of the disease, length of stay, ischemic etiology, heart rate interacting with atrial fibrillation (yes/no), systolic blood pressure interacting with left ventricular ejection fraction<50% (yes/no), radiologic evidence of pleural effusion (yes/no), peripheral artery disease (yes/no), serum sodium, serum brain natriuretic peptide, serum hemoglobin, serum urea, relative lymphocyte count, treatment with beta-blockers (yes/no) and oral anticoagulants (yes/no). Harrell′s C-statistics of the model was 0.752. The Gronnesby and Borgan test of goodness-of-fit showed a good model's calibration (P=.579). bModel 2: Multivariate Cox model adjusted by the same set of the covariates of model 1 plus main variables associated to statin prescription: previous treatment with statins (yes/no), history of dyslipidemia (yes/no), previous myocardial infarction (yes/no) and serum lipid levels. Harrell′s C-statistics of the model was 0.752. The Gronnesby and Borgan test of goodness-of-fit showed a good model's calibration (P=.555). cDose categories included: low dose (atorvastatin ≤10mg, simvastatin ≤20mg, pravastatin ≤40mg, lovastatin ≤40mg and fluvastatin ≤80mg) and medium-high dose (atorvastin ≥20mg and simvastatin ≥40mg). dInteraction P-value refers to the interaction between treatment with statins and CA125-binary status.
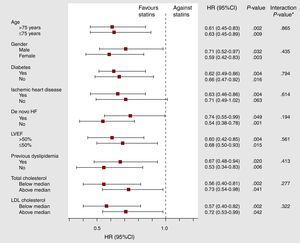
Furthermore, subgroup analyses revealed that this prognostic benefit of statins (in patients with high CA125 values) was uniform in the most representative subset of patients with AHF, such as those >75 years old, females, LVEF<50%, ischemic etiology, diabetes mellitus, and previous admission for AHF, even in patients with no previous dyslipidemia and total cholesterol and LDL cholesterol below the median (Figure 3).
Figure 3. Adjusted hazard ratio for the effect of statins on total mortality across main subgroups of patients with carbohydrate antigen 125 >35 U/mL. 95%CI, 95% confidence interval; HF, heart failure; HR, hazard ratio; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction.*Interaction P-value refers to the interaction between treatment with statins and each variable identifying the clinical subgroups.
The absolute association of statins with total mortality across CA125-binary status was evaluated by estimating an adjusted absolute risk difference (and NNT) at 3 and 6 months and 1, 3 and 5 years after admission (Table 2). In the group with CA125 >35 U/mL there was a consistent association of statins with mortality along the entire follow-up, and independently of the baseline hazard function for mortality; no association was shown in those patients belonging to the group of CA125 ≤35 U/mL. The equivalent translation to NNT is depicted in Table 2. Overall, treatment with statins in this population of patients with AHF was associated with 1 less death for every 7 to 14 patients treated, depending on the time at which it was evaluated.
Table 2. Adjusted Absolute Risk Difference and Number Needed to Treat for the Effect of Statins on Mortality, Among Carbohydrate Antigen 125-binary Status at the Specified Time Points.
| Time points | CA125 > 35 U/mL | CA125≤ 35 U/mL | ||
| Adjusted ARD (95%CI) | NNT (95%CI) | Adjusted ARD (95%CI) | NNT (95%CI) | |
| 3 months | 0.08 (0.03 to 0.12) | 13.1 (8.3 to 30.9) | −0.01 (−0.06 to 0.04) | −88.6 (27.5 to −17.0) |
| (P=.001) | (P=.642) | |||
| 6 months | 0.07 (0.02 to 0.12) | 13.8 (8.1 to 46.8) | −0.004 (−0.06 to 0.06) | −270.9 (18.2 to −16.0) |
| (P=.005) | (P=.902) | |||
| 1st year | 0.11 (0.05 to 0.17) | 9.3 (6.0 to 20.6) | 0.01 (−0.07 to 0.08) | 113.7 (11.9 to −15.1) |
| (P<.001) | (P=.819) | |||
| 3rd year | 0.11 (0.03 to 0.19) | 9.2 (5.3 to 35.9) | −0.01 (−0.12 to 0.09) | −73.9 (10.6 to −8.2) |
| (P=.008) | (P=.806) | |||
| 5th year | 0.14 (0.05 to 0.23) | 7.1 (4.3 to 19.7) | −0.004 (−0.13 to 0.12) | −232.5 (8.2 to −7.6) |
| (P=.002) | (P=.947) | |||
ARD, absolute risk difference; CA125, carbohydrate antigen 125; NNT, number needed to treat.
During follow-up, 418 cardiovascular deaths were registered (77.1%). Similar to all-cause mortality, those patients treated with statins and high levels of CA125 showed a marked decrease in the cumulative incidence function for cardiovascular mortality as compared to: a) non-statin-treated patients with CA125 >35 U/mL, and b) patients with normal CA125 values treated or not with statins (no significant difference in the cumulative incidence of cardiovascular death across statin therapy), as illustrated in supplementary material annex 3. Failure probabilities according to cause of death showed homogeneous mortality reduction for statin-treated patients with CA125 >35 U/mL for the most important causes of cardiovascular death (progressive HF and sudden death) and also for non-cardiovascular death (Table 3).
Table 3. Failure Probabilities According to Cause of Death.
| Time (year) | CA125≤35 U/mL No statins (n=258) | CA125≤35 U/mL Statins (n=171) | CA125>35 U/mL No statins (n=509) | CA125>35 U/mL Statins (n=284) | Total (n=1222) | ||||
| Died, (No.) | 1-KM | Died, (No.) | 1-KM | Died, (No.) | 1-KM | Died, (No.) | 1-KM | Died, (No.) | |
| Cardiovascular-death (n=418) | |||||||||
| 1 | 38 | 0.1575 | 22 | 0.1479 | 134 | 0.2825 | 49 | 0.1826 | 243 |
| 2 | 52 | 0.2312 | 33 | 0.2348 | 168 | 0.3761 | 64 | 0.264 | 317 |
| 3 | 65 | 0.3238 | 44 | 0.3616 | 189 | 0.4549 | 77 | 0.3664 | 375 |
| 4 | 72 | 0.3963 | 46 | 0.3928 | 203 | 0.5331 | 82 | 0.4257 | 403 |
| 5 | 73 | 0.4107 | 47 | 0.4217 | 209 | 0.5778 | 83 | 0.4417 | 412 |
| 6 | 73 | 0.4107 | 48 | 0.4743 | 210 | 0.5947 | 84 | 0.4789 | 415 |
| Progressive heart failure death (n=194) | |||||||||
| 1 | 18 | 0.0754 | 11 | 0.0741 | 83 | 0.1799 | 27 | 0.1029 | 139 |
| 2 | 22 | 0.099 | 13 | 0.0924 | 93 | 0.2136 | 33 | 0.1382 | 161 |
| 3 | 26 | 0.1332 | 15 | 0.119 | 98 | 0.2371 | 39 | 0.1954 | 178 |
| 4 | 29 | 0.1729 | 16 | 0.1416 | 103 | 0.278 | 39 | 0.1954 | 187 |
| 5 | 29 | 0.1729 | 16 | 0.1416 | 105 | 0.2998 | 40 | 0.2178 | 190 |
| 6 | 29 | 0.1729 | 16 | 0.1416 | 106 | 0.3278 | 41 | 0.2699 | 192 |
| Sudden death (n=55) | |||||||||
| 1 | 6 | 0.0268 | 3 | 0.0223 | 20 | 0.0499 | 8 | 0.0325 | 37 |
| 2 | 6 | 0.0268 | 4 | 0.0313 | 24 | 0.0649 | 9 | 0.0407 | 43 |
| 3 | 8 | 0.0489 | 9 | 0.107 | 25 | 0.0701 | 10 | 0.0524 | 52 |
| 4 | 9 | 0.0631 | 10 | 0.1283 | 26 | 0.082 | 10 | 0.0524 | 55 |
| 5 | 9 | 0.0631 | 10 | 0.1283 | 26 | 0.082 | 10 | 0.0524 | 55 |
| 6 | 9 | 0.0631 | 10 | 0.1283 | 26 | 0.082 | 10 | 0.0524 | 55 |
| Non-cardiovascular death (n=124) | |||||||||
| 1 | 12 | 0.0547 | 5 | 0.0330 | 28 | 0.0730 | 6 | 0.0253 | 51 |
| 2 | 19 | 0.0958 | 11 | 0.0952 | 38 | 0.1106 | 19 | 0.1073 | 87 |
| 3 | 24 | 0.1356 | 12 | 0.1129 | 46 | 0.1582 | 20 | 0.1167 | 102 |
| 4 | 25 | 0.1461 | 14 | 0.1524 | 52 | 0.2105 | 22 | 0.1497 | 113 |
| 5 | 25 | 0.1461 | 15 | 0.1877 | 55 | 0.2529 | 24 | 0.1998 | 119 |
| 6 | 26 | 0.1802 | 15 | 0.1877 | 55 | 0.2529 | 24 | 0.1998 | 120 |
CA125: carbohydrate antigen 125; KM: Kaplan Meier.
In multivariable analysis, while accounting for non-cardiovascular death as a competing event, the interaction between CA125 and statins was borderline significant (P-value for interaction=.051). Constrained by this interaction, those patients displaying CA125 >35 U/mL and treated with statins showed a significant 38% risk reduction (HR=0.62, 95%CI: 0.47-0.81; P<.001), whereas statin therapy failed to show any benefit in those with normal CA125 values (HR=0.97, 95%CI: 0.66-1.41; P=.703), as shown in supplementary material annex 2.
DISCUSSIONIn the present study, we found that in patients with AHF the association of statins with long-term mortality was differentially determined by CA125 serum levels. In contrast to patients with normal CA125 values (≤35 U/mL), where no association was observed, in patients exhibiting high CA125 values (>35 U/mL) statin treatment was associated with a significant 35% reduction in total mortality. The consistency of this differential prognostic effect was confirmed despite a thorough covariate adjustment (including lipid levels), and also observed among the most important clinical subgroups, including patients with non-ischemic HF etiology and those with preserved systolic function. Moreover, the beneficial association of statins observed in the subgroup of patients with CA125 >35 U/mL was also endorsed by a significant difference in absolute risk shown along the entire follow-up.
Although the findings of subgroup analyses should be cautiously interpreted, we believe that our main finding provides additional support to the results of a recent analysis of the CORONA trial,6 where the main prognostic benefit of statins in HF was observed only in patients with elevated proinflammatory activity.8.
Carbohydrate Antigen 125 as a Marker of Inflammation and Its Theoretical Advantages Over Other Inflammatory BiomarkersThere is increased evidence underlying the prognostic role of CA125 in advanced HF.11, 12 Although the exact mechanism for this association has not yet been determined, it is believed to be related to complex pathophysiological processes linking inflammation and systemic congestion.22 For instance, in AHF setting, CA125 levels did show to correlate with proinflammatory cytokines and low relative lymphocytes count.9, 10 Furthermore, in vitro studies have shown that proinflammatory cytokines such as interleukin-1, tumor necrosis factor-α and lipopolysaccharide can stimulate human mesothelial cells to increase secretion of CA125.23.
Compared to other inflammatory biomarkers not yet ready for routine clinical use, CA125 offers some meaningful potential advantages such as wide availability in routine clinical practice, standardized measurement, low cost and pharmacokinetics reliability. It is well known that most proinflammatory cytokines exhibited high temporal variability for reasons that are out of the scope of this work (unstandardized measure techniques, pulsatile release, short half-lives, etc.).24 In contrast, CA125 kinetic displays a mean span-life higher than 1 week,13 even much longer than 18-20h of CRP plasma half-life,25 which allows us to postulate CA125 as a reliable biomarker for baseline identification of heightened inflammatory activity. On the other hand, clinical observations have shown that serial changes of CA125 over time are kept within values that reflect the patient's clinical status and prognosis.26, 27 Based on all of these attractive properties, we believe that this glycoprotein may have a potential role for monitoring and guiding therapy in HF, either as stand-alone or combined with natriuretic peptides. Not surprisingly, the main clinical use of this biomarker, outside HF setting, is for monitoring therapy in ovarian cancer.28.
Pleiotropic Effects of Statins. A Matter of DebateSeveral mechanisms have been proposed to explain the unexpected (and disappointing) results of the CORONA6 and GISSI7 trials. On one hand, lipoproteins have been shown to neutralize the endotoxin inflammatory response, and thus, by lowering them with statins, the bioactivity of bacterial endotoxins may potentially increase.2, 29 Statins also reduce plasma levels of ubiquinone, a coenzyme in mitochondrial respiration, which at least on theoretical ground, may affect myocardial function.2 On the positive side, however, a large number of studies have shown the effects of statins in reducing inflammatory markers (CRP, tumor necrosis factor-α, interleukin-1 and interleukin-6) including patients with HF.30 The longstanding success of statins in HF speaks in favor of the hypothesis that the net effect of these opposite forces goes in favor of the anti-inflammatory effect. The clinical benefit of statin therapy based on inflammatory status is also reinforced in general population by the results of JUPITER trial, where in healthy persons without dyslipidemia but with elevated CRP levels, rosuvastatin significantly reduced the incidence of major cardiovascular events.31 Nevertheless, generating more debate, a recent substudy of the Heart Protection Study showed that reducing LDL cholesterol with simvastatin reduces the risk of major vascular events to a similar extent irrespective of CRP concentration.32.
In AHF, elevated inflammatory activity interacts through complex pathophysiological pathways with systemic congestion,22 and is thought to contribute to the high morbidity and mortality observed after the first months following the acute episode. Several observational studies have reported better survival among statin treated patients in the setting of AHF3, 5 and in patients with severe bacterial infections,33 where inflammation plays a crucial pathogenic role. We believe, however, that AHF represents an inhomogeneous clinical entity, not only in aetiology and LVEF, but also in levels of systemic inflammation. AHF patients, especially those with over-activity of the immune system constitute a plausible target to further investigate the statin therapeutic effects.
LimitationsThere are a number of potential limitations in this study: a) this is a single-center observational study where, by design, different types of bias and residual confounding may be operating; b) the adjudication of specific cause of death was mainly done through patients’ chart review which, to some extent, may introduce some degree of endpoint misclassification; c) with the present data, we cannot address the complex pathogenic mechanisms that may be operating between statins, HF and mortality; d) knowing that the indication for statins in our cohort was for the treatment of dyslipidemia, we are unable to discard that our results may be confounded by this indication; e) due to the small size of our cohort and the many different types and doses of statins, it was not possible to determine whether a dose-response or a class effect was present; f) we did not evaluate either the adherence to statin therapy or the impact that serial changes in CA125 would have had on our results, and g) the lack of measurement of CRP in all patients precludes to include this biomarker in this analysis.
CONCLUSIONSIn conclusion, in this hypothesis generating study, statin therapy in AHF was associated with a decreased risk of mortality, but only for those patients with CA125 levels >35 U/mL. These results provide a rationale to further investigate the therapeutic role of statins among HF patients with evidence of systemic inflammation as evidenced by elevated CA125 levels in a more controlled scenario.
FUNDINGThis study was supported by a grant from Conselleria de Sanitat (Dirección General de Ordenación, Evaluación e Investigación Sanitaria para el fomento de la investigación en la Comunidad Valenciana: AP-132/08) and by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, RED HERACLES RD06/0009/1001 (Madrid, Spain).
CONFLICT OF INTERESTSDr. Gregg Fonarow has research from the National Institues of Health, received honorarium from GlaxoSmithKline, Medtronic, Novartis, and Pfizer, and consulted for GlaxoSmithKline, Merck, Novartis, and Pfizer.
.
Appendix A. Supplementary materialSupplementary material associated with this article can be found in the online version available at doi:10.1016/j.rec.2011.05.033.
Appendix A. Supplementary materialReceived 15 March 2011
Accepted 29 May 2011
Corresponding author: Servicio de Cardiología, Hospital Clínico Universitario, Avda. Blasco Ibáñez 17, 46010 Valencia, Spain. yulnunez@gmail.com