High endothelin-1 (ET-1) levels have been linked to poor clinical outcomes after ST-segment elevation myocardial infarction (STEMI). Vasoconstriction of the coronary microcirculation seems to be the underlying mechanism. The aim of the study was to assess the effect of ET-1 on microvascular integrity, infarct size, left ventricular ejection fraction (LVEF) and myocardial salvage in evolving myocardial infarction (MI).
MethodsWe measured ET-1 levels acutely (6–24h) in 127 patients presenting with their first STEMI. Contrast-enhanced cardiac magnetic resonance (ce-CMR) was performed in 94 patients within 1 week to assess microvascular obstruction (MO), infarct size and LVEF. A myocardial salvage index (MSI) was defined as the percentage of at-risk angiographic area without necrosis on the ce-CMR.
ResultsMean age was 60.9 (11.8) years and 98 (77%) were males. Median ET-1 level within the first 24h was 6.8 pg/mL (25th–75th percentile range: 5.4–8.5pg/mL). Patients with ET-1 concentrations over the median presented higher percentage of MO (77.7% for ET-1>6.8pg/mL vs. 16.6% for ET-1≤6.8pg/mL, P<.001) and lower MSI values (13.8 (26%) for ET-1>6.8pg/mL vs. 37.4 (26%) for ET-1≤6.8pg/mL, P=.02). ET-1 levels did not show a significant association with infarct size (P=.11) and LVEF (P=.16). Multivariate analysis found ET-1 to be a significant predictor of MO (OR=2.78; CI 95% 1.16–6.66; P=.021) and MSI≤Percentile 25 (OR=1.69, CI 95% 1.01–2.81; P=.04).
ConclusionsHigh ET-1 levels after myocardial infarction are associated with the presence of microvascular obstruction and lower myocardial salvage index.
Keywords
Reperfusion injury after primary percutaneous coronary intervention (PCI) has been a matter of intensive study in recent years due to its high incidence1 (around 40%) and unquestionable implications for poor prognosis following acute ST-segment elevation myocardial infarction (STEMI).2,3,4,5,6
Reperfusion injury is a multifactorial process that focuses on coronary microcirculation.7,8,9 Coronary endothelium is the most important regulator at this level. Endothelin-1 (ET-1), released by endothelial cells, is the most potent endogenous vasoconstrictor yet identified,10,11 as well as an important modulator of neutrophil function,9,12,13 and stimulates surface expression of adhesion molecules.14
ET-1 levels increase 3–4h after the onset of STEMI, peak within the first 24h and remain elevated after 48h.15 High ET-1 values after PCI have been linked to poor prognosis, including higher 30-day mortality.16 Microvascular vasoconstriction together with leukocyte adhesion seems to be the underlying mechanism of ET-1 injury, although this has only been established in animal models.17,18 In humans, evidence of microvascular injury has been obtained as angiographic scores, primarily by indirect methods such as the thrombolysis in myocardial infarction (TIMI) grade flow and myocardial blush grade (MBG),19 and more recently by cardiac magnetic resonance.20
Contrast-enhanced cardiac magnetic resonance (ce-CMR) allows precise characterization of several cardiac parameters, including the presence of microvascular obstruction (MO) and the percentage of myocardial salvage. The aim of the present study was to make a comprehensive evaluation of the relationship between ET-1 levels and ce-CMR derived parameters of myocardial damage like MO, infarct size, myocardial salvage and left ventricular (LV) function. A secondary objective was to evaluate the linkage between ET-1 concentrations and 30-day mortality, Killip class on admission and angiographic reperfusion outcomes.
MethodsThis was a prospective and single-center study. The study protocol was approved by the ethics committee of our institution following the ethical guidelines of the 1975 Declaration of Helsinki. All patients gave written informed consent before inclusion.
Study PopulationBetween June 2007 and December 2008, we prospectively screened 151 consecutive patients who underwent primary or rescue PCI for their first STEMI. Inclusion criteria were prolonged chest pain (>30min) and ST-segment elevation >1mm in two or more adjacent leads within the first 24h after onset of symptoms. Exclusion criteria included recent surgical or trauma history, renal insufficiency (creatinine >1.5mg/dl), malignancy, acute or chronic inflammatory disease or previous infection.
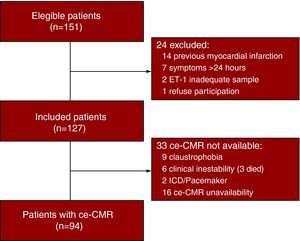
Of the 151 patients screened, 127 subjects were included. A complete ce-CMR study was available in 94 patients (Figure 1Fig. 1).
Figure 1. Flow diagram. ET-1, endothelin-1; ce-CMR, contrast-enhanced cardiac magnetic resonance; ICD, implantable cardiac defibrillator.
Blood Sampling and Laboratory AssayTwo blood samples for ET-1 measurements were obtained from a peripheral vein, the first between 4 and 24h (mean 10.8 (7.5)h) from symptoms onset and the second at ∼48h (mean 45 (8.1)h). If not specified, the first sample was the reference for all the analysis.
Samples were decanted into EDTA (Ethylene Diamine Tetraacetic Acid) tubes, centrifuged at 3000rpm for 15min and stored at −80°C until analysis. Plasma ET-1 levels were determined by radio-immunoassay using rabbit anti-ET-1 antibody (Peninsula Laboratories Inc., San Carlos, CA, USA). All measurements were repeated and averaged. The mean intra-assay coefficient of variance was 6.1%.
Percutaneous Coronary Intervention and Angiographic AnalysisA transradial or transfemoral artery 6F approach was performed. Unfractionated heparin was administered at a dose of 60IU/kg and a loading dose of 300mg of Clopidogrel was administered during PCI. Our protocol includes administration of abciximab prior to PCI if no contraindications; the decision on use of thrombectomy devices, direct stenting or intracoronary adenosine administration was left to the angiographer.
Angiographic assessment was always performed independently by two experienced angiographers and included TIMI flow grading,21 MBG,22 Rentrop collateral grade23 and at-risk angiographic area, determined by Bypass Angioplasty Revascularization Investigation (BARI) score.24,25 Suboptimal reperfusion was defined as post-procedural TIMI flow≤2 or MBG≤2 despite TIMI 3 flow.
Cardiac Magnetic Resonance AnalysisSubjects were imaged in a 1.5T clinical scanner (CV Signa, GE, Milwaukee, WI, USA) equipped with cardiac-dedicated software and a 4-element cardiac phased-array surface coil. In addition to the standard 2, 3 and 4 chamber views, a stack of sequential short axis images every 10mm with no gap was obtained from the LV base to the apex to achieve full LV coverage. All images were acquired during breath-holding and were electrocardiographically gated. Functional assessment of the left ventricle was performed using a standard cine steady-state free precession sequence (FIESTA).
A standard segmented inversion-recovery fast gradient-echo pulse sequence was prescribed in identical positions 10–15min after intravenous administration of gadodiamide (Omniscan, Amersham Health, Madrid, Spain) at a dose of 0.2mmol/kg. Mean inversion time, adjusted to null normal myocardium, was 200–220ms. Matrix size was set to 256 and the mean field of view was 360mm, resulting in a typical voxel size of 1.4mm×1.4mm×10mm. All cardiac magnetic resonance (CMR) images were cropped, interpolated by a factor of 3 and anonymized for analysis by an independent, experienced cardiologist blinded to all clinical and angiographic data.
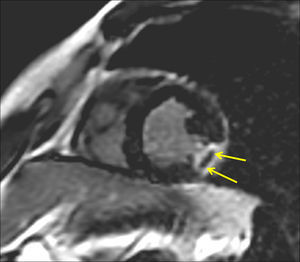
Microvascular obstruction was defined as any area of late hypo-enhancement surrounded by hyper-enhancement on ce-CMR images (Figure 2Fig. 2).
Figure 2. Ce-CMR late enhancement in a patient with an ET-1 value≥median on admission demonstrating transmural scar of the lateral wall with MO in the short axis. The arrows show a clear area of late hypo-enhancement surrounded by hyper-enhancement, which defines the presence of MO. ce-CMR, contrast-enhanced cardiac magnetic resonance; ET-1, endothelin-1; MO, microvascular obstruction.
The infarcted myocardium, defined as areas of hyper-enhancement with signal intensity 2 standard deviations above that of normal remote myocardium, was manually planimetered on short-axis contrast-enhanced images by an experienced observer. The total infarct area on sequential short-axis slices was divided by the total LV myocardial volume to calculate the infarct size as a percentage of the LV myocardial volume. Infarct size by ce-CMR was subtracted from the estimated at-risk angiographic area to compute myocardial salvage, expressed as percentage of LV wall volume. To account for differences in the anatomic area at risk, a myocardial salvage index (MSI) was computed as follows: MSI=[area at risk by angiography−infarct size by ce-CMR]×100/area at risk by angiography, expressed as % of the area at risk.24,25
Using customized Image J (National Institutes of Health, Bethesda, MD, USA) tools, the endocardial and epicardial borders on sequential short axis cine images were manually traced at end-systole and end-diastole to compute LV end-systolic and end-diastolic volumes as well as LV mass and LV ejection fraction (LVEF).
Statistical AnalysisThe results are expressed as mean (SD) or median, depending on normal distribution as assessed by the Shapiro–Wilks test. Comparisons between groups were performed using unpaired t test or Mann–Whitney U test for continuous variables and Chi-square or Fisher's exact test for categorical variables. Multivariate logistic regression analysis was conducted to identify whether ET-1, as a continuous variable, was independently associated with MO and myocardial salvage. Variables screened included basal characteristics (age, gender, cardiovascular risk factors) as well as time to reperfusion, Rentrop collateral grade, area at risk, and use of abciximab, adenosine and aspiration devices. Variables showing a trend towards (P<.1) or a significant value (P<.05) at univariate analysis were included in the model. Additionally, the authors included the use of abciximab, adenosine and aspiration devices in the MO model, since these are recognized modulators of reperfusion injury.26,27,28 For additional assessment of ET-1 value with regard to MO, receiver operating characteristic (ROC) curves were generated and the area under the curve (AUC) was calculated. Results were considered statistically significant at a P-value <.05. Statistical analyses were done using SPSS package v14.0 (Chicago, IL, USA).
Results Patient CharacteristicsThe clinical characteristics of the study population are shown in Table 1. Primary PCI was performed in 109 subjects (86%) and rescue PCI in 18 subjects (14%). Mean symptoms-to-balloon time was 282±189 and 412±356min for primary and rescue PCI, respectively. In rescue PCI cases, mean symptoms-to-lysis time was 230±260min. The angiographic characteristics of the cohort are listed in Table 2. Suboptimal reperfusion was observed in 43% of patients. Four patients (3%) died within the first 30 days after admission due to cardiogenic shock in 3 cases and refractory heart failure in 1 case. Microvascular obstruction on ce-CMR images was observed in 48% of patients.
Table 1. Clinical Characteristics of Patients (n=127).
| Age (years) | 60.9 (11.8) |
| Males (%) | 77.2 |
| Hypertension (%) | 51.2 |
| Hypercholesterolemia (%) | 37.0 |
| Diabetes (%) | 17.3 |
| Smokers (%) | 49.6 |
| Previous PCI | 8.7 |
| Previous CABG | 0.8 |
| Systolic blood pressure (mmHg) | 129.3 (31.6) |
| Heart rate (bpm) | 77.2 (19.1) |
| AMI location: Anterior (%) | 47.2 |
| Time: Symptoms-balloon (min) | 339 (276) |
| Killip at admission | |
| I | 91.2 |
| II | 4.8 |
| III | 0.8 |
| IV | 3.2 |
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention.
Table 2. Angiographic and Procedure Data (n=127).
| Culprit vessel | |
| LAD | 42.5% |
| LCX | 18.1% |
| RCA | 37.7% |
| LIn | 1.7% |
| Number of diseased vessels | |
| 1 | 71.4% |
| 2 | 23.0% |
| 3 | 5.6% |
| Pre-PCI TIMI | |
| 0 | 58.7% |
| 1 | 6.6% |
| 2 | 15.7% |
| 3 | 19.0% |
| Post-PCI TIMI | |
| 1 | 0.8% |
| 2 | 5.6% |
| 3 | 93.6% |
| Post-PCI MBG | |
| 1 | 7.6% |
| 2 | 32.2% |
| 3 | 60.2% |
| Pre-PCI Rentrop collateral grade | |
| 0 | 31.0% |
| 1 | 55.0% |
| 2 | 11.0% |
| 3 | 3.0% |
| Treated vessels | |
| 1 | 92.1% |
| 2 | 7.1% |
| 3 | 0.8% |
| Abcximab | 64.0% |
| Aspiration devices | 21.0% |
| Intracoronary adenosine | 22.0% |
| Direct stenting | 49.0% |
LAD, left anterior descending; LCX, left circumflex; LIn, left intermediate; MBG, myocardial blush grade; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIMI: thrombolysis in myocardial infarction.
Median ET-1 level within the first 24h was 6.8pg/mL (25th–75th percentile range: 5.4–8.5pg/mL). As shown in Table 3, patients with ET-1 concentrations over the median presented higher percentage of MO (77.7% for ET-1>6.8 pg/mL vs. 16.6% for ET-1≤6.8pg/mL, P<.001) and lower MSI values (13.8 (26)% for ET-1>6.8pg/mL vs. 37.4 (26)% for ET-1≤6.8pg/mL, P=.02). ET-1 values over the median were also associated with worse admission Killip class and post-PCI MBG, and higher 30-day mortality. ET-1 levels did not vary significantly with usage of abcximab, adenosine, aspiration devices or direct stenting.
Table 3. Univariate Analysis of Endothelin-1 Association With Cardiac Magnetic Resonance Variables, Angiograpgic Area at Risk, Admission Killip, Myocardial Blush Grade and 30-Day Mortality.
| Endothelin-1 ≤6.8pg/mL | Endothelin-1 >6.8pg/mL | P value | |
| Microvascular obstruction (%) | 16.6 | 77.7 | P<.001 |
| Myocardial salvage index (%) | 37.4 (26.5) | 13.8 (26.2) | P=.02 |
| Infarct size (%) | 19.1 (11.2) | 26.0 (11.3) | P=.11 |
| LVEF (%) | 46.7 (10.0) | 42.3 (8.1) | P=.16 |
| BARI area at risk (%) | 29.9 (9.1) | 29.9 (10.6) | P=.98 |
| Killip on admission≥II (%) | 2.0 | 13.0 | P=.04 |
| MBG<3 (%) | 26.5 | 77.7 | P=.005 |
| 30-day mortality (%) | 0 | 7.4 | P=.03 |
Abbreviations: BARI, bypass angioplasty revascularization investigation; LVEF, left ventricular ejection fraction; MBG, myocardial blush grade.
The multivariate analysis included ET-1 levels, sex, aspiration devices, abcximab, and adenosine usage for the MO model and ET-1 levels, sex, diabetes and aspiration devices for the MSI model. ET-1 was found to be the only significant predictor of MO (OR=2.78; CI 95% 1.16–6.66; P=.021) and MSI≤Percentile 25 (OR=1.69, CI 95% 1.01–2.81; P=.04).
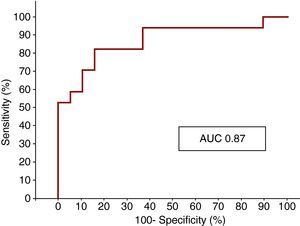
The ROC curve analysis showed that ET-1 level on admission was a strong indicator of MO, with an AUC of 0.87 (Figure 3).
Figure 3. ROC curve showing the sensitivity and specificity of endothelin-1 for microvascular obstruction. AUC, area under the curve.
Endothelin-1 at 48hMedian ET-1 value within the first 48h after myocardial infarction onset was 5.92 pg/mL (25th–75th interquartile range: 5.0–7.6pg/mL). At 48h, ET-1 did not present significant association with MO (P=.24), MSI (P=.26), infarct size (P=.07), LVEF (P=.35), post-PCI MBG <3 (P=.08), admission Killip class (P=.127) and 30-day mortality (P=.20). In most cases (67%), baseline ET-1 levels were higher than in subsequent determinations; the opposite finding occurred less frequently (33% of cases) but was associated with higher 30-day mortality (0% vs. 7%; P=.04).
DiscussionOur results suggest an association between high ET-1 levels within the first 24h after STEMI and evidence of microvascular injury, as depicted by ce-CMR and angiographic variables.
In the context of the intense recent interest in reperfusion injury after primary PCI, the present study supports the reported association between higher ET-1 levels and reperfusion injury, and offers novel insights into the mechanisms of its deleterious effect on microcirculation and myocardial salvage. Despite a limited sample size, we found an association between ET-1 expression and several ce-CMR parameters linked to worse clinical outcomes.
Higher ET-1 levels were significantly associated with MO and lower MSI, but we were not able to find a significant association with infarct size, LVEF and BARI area at risk. A possible explanation for these findings is that ET-1 levels were more likely to be associated with the reperfusion outcomes of a specific area at risk. It would follow, then, that ET-1 levels could be higher in a smaller area at risk of STEMI (e.g., occluded distal right coronary artery) with severe reperfusion injury than in a larger area (e.g., proximal left anterior descending artery) without reperfusion damage.
As expected, suboptimal angiographic reperfusion and ce-CMR MO rate were very similar (43% and 48%, respectively) and did not differ from previous published data.29
In accordance with previous studies,17,30 high ET-1 levels were also related with higher incidence of congestive heart failure on admission and higher 30-day mortality.
Timing implications are also important, since ET-1 measurements were done within the first 24h after onset of acute myocardial infarction, allowing early and proper assessment of reperfusion outcomes with only 1 blood sample. As a result, specific treatment aimed at reducing reperfusion injury could be considered from the early stages of STEMI. Although clinical experience is still insufficient, animal models have associated endothelin receptor antagonists with significant reduction of ischemic-reperfusion injury after myocardial infarction31,32 and improvement in myocardial protection after cardiac surgery.33 Patients with admission Killip ≥2 could be candidates for these treatments due to the relationship between heart failure and higher ET-1. Further studies will be necessary to clarify whether patients with high ET-1 levels are better candidates for treatment with endothelin receptor antagonists.
According to our results, isolated ET-1 measurements at 48h after AMI should not be recommended, since no significant linkage with any of the ce-CMR, angiographic or clinical parameters was observed. However, a remarkable finding of the present study was the importance of serial ET-1 measurements, as subsequent increases over baseline levels may identify a particular subset of patients at risk for future adverse cardiac events. This finding is consistent with previous observations showing that this particular pattern of ET-1 expression is associated with increased rates of heart failure and persistent ischemia.14 Although the descending pattern was the most frequent in our series, we found, in agreement with previously published findings, that the ascending pattern was related to higher 30-day mortality.
In summary, the current study highlights the role of ET-1 as a novel marker of microvascular dysfunction. This suggests that ET-1 assessment should be considered for implementation in daily clinical practice, especially in high-risk STEMI patients.
LimitationsThere are several limitations to this study. First, without serial measurement of circulating ET-1 levels, we could not determine the real peak-value and time to peak ET-1 level. However, there were no significant differences in the mean ET-1 values within the first 24h. In addition, this flexible time interval represents an achievable target in daily practice. Second, ce-CMR was not performed in some patients due to clinical instability or technical problems. Although we were not able to assess microvascular integrity in these patients, we did not exclude them from the analysis of clinical and angiographic outcomes. Finally, some of the drugs or devices used during PCI (such as abciximab, intracoronary adenosine or aspiration devices) might have interfered with microvascular integrity. Their usage, left to the operator's decision, was more likely in patients with reperfusion injury. For instance, intracoronary adenosine is most often used in cases with slow flow or no reflow, and aspiration devices are commonly used in patients with a large thrombus burden who are at higher risk for distal embolization.
ConclusionsHigh ET-1 levels after myocardial infarction are associated with the presence of MO and lower MSI.
FundingProject funded by the Spanish Society of Cardiology and the Hospital Clinic of Barcelona.
Conflicts of InterestThe authors state that they have no conflicts of interest.
Received 31 May 2010
Accepted 29 July 2010
Corresponding author: Servicio de Cardiología, Instituto del Tórax, Hospital Clínic de Barcelona, Universidad de Barcelona, Villarroel 170, Barcelona 08036, Spain. freixa@clinic.ub.es