To determine the effect of opening an on-site diagnostic catheterization facility on 30-day and 2-year mortality rates in patients with myocardial infarction (MI).
MethodsThe study included 1539 consecutive MI patients aged 25–74 years who were recruited before and after the catheterization laboratory opened in 1998: during 1995–1997 and 1999–2003, respectively.
ResultsThe 641 consecutive MI patients recruited in 1995–1997 had worse 30-day mortality than the 898 recruited between 1999–2003 (11.2% versus 6.35%, respectively; P=.001). The number of coronary angiographies and percutaneous coronary interventions (PCIs) carried out was greater in the second period (19.4% versus 3.3%, and 54.8% versus 23.0%, respectively; P<.001). Two-year survival curves were significantly better in the second period for all-cause and cardiovascular death. The adjusted odds ratio for death at 30 days was 0.58 (95% confidence interval [CI] 0.36–0.95) for the second period compared with the first and the adjusted hazard ratio for cardiovascular death at 2 years for patients still alive at 30 days was 0.62 (95%CI 0.39–0.99). After adjustment for the prescription of statins, angiotensin-converting enzyme inhibitors, beta-blockers and antiplatelet drugs at discharge, the effect observed at 2 years was no longer significant.
ConclusionsOpening a new on-site diagnostic catheterization unit significantly increased the 30-day survival of MI patients. However, the increase in 2-year survival of 30-day survivors observed was largely explained by the implementation of better secondary prevention.
Keywords
Although mortality rates are lower in Spain than in northern Europe, cardiovascular disease (CVD) remains the principle cause of death in the country. Consequently, it is generally accepted that increased aging of the population and better survival after a first event will result in a rising workload for departments of cardiology.1,2,3
Evidence from clinical trials and retrospective studies show that coronary angiography and coronary angioplasty improve both the management of patient with acute coronary syndrome (ACS) and the outcomes obtained.4 However, the use of coronary angiography in patients with myocardial infarctions (MI) can vary between 15% and 50% without affecting morbidity and mortality at 6 months, provided that urgent indications are dealt with quickly.5 On the other hand, when its use varies substantially between centers (e.g. 6% to 93%), there is a significant effect on mortality.6 Having a cardiac catheterization laboratory on site is the critical factor that determines the use angiography and angioplasty.5,7,8 Therefore, the opening a new diagnostic coronary catheterization unit in a center that previously had to refer all its patients to a distant tertiary hospital provides a good opportunity for a prospective analysis of the benefits of an on-site unit.
The objective of the present study was to determine whether the availability of a new diagnostic cardiac catheterization facility in a tertiary hospital had an effect on 30-day and 2-year cardiovascular mortality rates in consecutively admitted patients with MI. Given that it has been shown over the last decade that the use of new therapies such as thrombolysis,9 antiplatelet drugs,9,10 beta-blockers,11 angiotensin-converting enzyme (ACE) inhibitors,12 and statins,13 has improved the prognosis of ACS patients who present with ST-elevation leading to MI, a second objective of the study was to determine whether the patient's drug regime at discharge contributed to any differences in outcome observed.
Methods PatientsThe Girona Heart Registry (Registre Gironí del cor; REGICOR) was established in 1978 and contains the records of all consecutive patients with MI aged 25 to 74 years who live in the Girona catchment area.14
Time periodsWe included patients registered during two distinct time periods during the era of invasive cardiology: 641 from 1995–1997 and 898 from 1999–2003. The diagnostic catheterization laboratory opened in early 1998; we omitted this start-up year from the analysis.
Measurements and Patient ManagementThe following patient data were collected prospectively: age, sex, smoking status, history of hypertension, diabetes and angina, type of acute coronary syndrome, and final ECG MI findings. Disease severity was determined from the degree of ventricular dysfunction (e.g. acute pulmonary edema or cardiogenic shock) and the presence of ventricular arrhythmias (e.g. fibrillation or tachycardia) that required immediate treatment. Treatment variables, such as the use of thrombolysis, antiplatelet drugs, coronary angiography, percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery, were also recorded. Prescription of antiplatelet drugs, statins, ACE inhibitors, and beta-blockers at discharge was noted to assess the potential influence of drug treatment on 2-year outcomes.
Patients were treated according to the guidelines in force at the time, which did not differ substantially in the two periods studied.15,16 Consequently, in these two periods, the use of coronary angiography was restricted to patients with heart failure, ventricular dysfunction, post-acute myocardial infarction (AMI) angina and residual ischemia.
DefinitionsThe MI was categorized as Q-wave, non-Q-wave or unclassifiable MI from the discharge ECG if a patient presented with chest pain lasting more than 20minutes on admission and had exhibited characteristic ECG changes in serial recordings. To standardize diagnoses over the two time periods studied, any abnormal increase in creatine kinase level was taken into account. The troponin level was not considered because the marker had not been introduced at the time of the earlier study period.
The diagnosis of re-infarction was based on the occurrence of typical ECG changes, defined using the same criteria as for the initial MI, along with a second increase in creatine kinase level following the peak associated with the MI present at first admission.
Criteria for post-MI angina were the recurrence of angina symptoms with ECG changes 24hours to 30 days after MI symptom onset, without an increase in cardiac enzyme levels.
Unstable angina during follow-up (i.e. 30 days to 2 years after MI symptom onset) was diagnosed from the recurrence of angina symptoms accompanied by ECG changes in serial recordings, with an increase in cardiac enzyme levels below the limit previously described for MI diagnosis. The definition of MI used during follow-up was the same as for the first MI.
Follow-upPatients still alive after 30 days were followed up by telephone 2 years later and data on the cohorts were crosschecked with the official Catalan Department of Health mortality register. Data were also obtained by reviewing the hospital medical records of all patients reported to have been hospitalized for an apparent cardiac condition.
The principal end-points were 30-day and 2-year cardiovascular mortality. We also recorded all-cause mortality (during the acute phase, that is in the first 30 days after symptom onset, all deaths were considered to be of cardiac origin), new MIs and admission for unstable angina.
Sample Size and Statistical AnalysesOur study was sufficiently powered (i.e. 86%) such that a hazard ratio (HR) for mortality ≤0.65 for the second period found in a two-sided test would be statistically significant (P<.05). It was assumed that 2-year mortality would be >15% in the first period, that the first/second ratio would be 40%, and that the correlation of the time period variable with potential confounders would be <0.3.
Differences between the time periods and between patients who did or did not experience an event within 30 days or, among 30-day survivors, within 2 years were assessed using a chi-squared test for categorical variables and Student's t test for continuous variables, or the non-parametric equivalents, as appropriate.
The adjusted odds ratio (OR) for an event within 30 days in the second time period was calculated using a logistic model, and the HR for mortality at 2 years in 30-day survivors was estimated using a Cox model. Any demographic, comorbidity, clinical or severity variables associated with an, at least, marginally significant difference (i.e. P≤.15) on bivariate analysis between the time periods or between patients who did and did not experience events at 30 days or 2 years and any variables that were considered important on the basis of clinical judgment were included as potential confounders (e.g. sex, age and diabetes). Severity variables (i.e. acute pulmonary edema or cardiogenic shock), which could be interpreted as intermediate mechanisms of death, were included in separate models along with the above-mentioned variables. The final model for the 2-year follow-up of 30-day survivors included statin, ACE inhibitor, antiplatelet and beta-blocker prescription at discharge.
Survival curves were derived using the Kaplan–Meier method and compared using Mantel–Cox statistics. Calculations were carried out using the R statistical package.
ResultsDuring 1995–1997, there were 3345 admissions to the cardiology service, of which 919 were for an AMI. Of these, 783 were for a first AMI in individuals resident in our catchment area. During 1999–2003, there were 4629, 1332 and 1108 cases, respectively.
The demographic characteristics and cardiovascular risk factors of patients admitted during 1995–1997 and 1999–2003 are shown in Table 1. Patients in the more recent period were younger, were more likely to be current smokers, and more often had a diagnosis of hypertension, hypercholesterolemia, or non-Q-wave MI. The proportion who underwent thrombolysis or CABG surgery was similar, but the number of coronary catheterizations and PCIs increased with the availability of the on-site catheterization laboratory. Delays in performing coronary angiography, PCI and CABG surgery were significantly shorter in the second time period. The proportion taking statins, ACE inhibitors, beta-blockers and antiplatelet drugs at discharge was higher in the second period.
Table 1. Demographic and Clinical Characteristics of Myocardial Infarction Patients, by Period of Admission Without and With an On-Site Catheterization Laboratory (1995-1997 and 1999-2003).
| 1995–1997 | 1999–2003 | P-value | |
| Patients, n | 641 | 898 | |
| Age, years * | 63.7 (11.9) | 60.7 (10.9) | <.001 |
| Percentage women | 22.0% | 20.3% | .411 |
| Risk factor history | |||
| Hypertension | 46.5% | 52.2% | .029 |
| Diabetes | 27.3% | 26.0% | .568 |
| Hypercholesterolemia | 41.2% | 52.0% | <.001 |
| Current smokers | 35.9% | 43.6% | .003 |
| ECG myocardial infarction findings | |||
| Non-Q-wave | 16.5% | 26.7% | |
| Inferior | 42.7% | 37.0% | |
| Anterior | 35.7% | 31.7% | |
| Unclassifiable | 5.0% | 4.6% | <.001 |
| Type of acute coronary syndrome | |||
| ST-elevation | 82.5% | 74.4% | |
| Non-ST-elevation | 12.3% | 20.8% | |
| Unclassifiable | 5.2% | 4.8% | <.001 |
| Procedure used during the first 30 days | |||
| Thrombolysis | 41.5% | 40.2% | .612 |
| Coronary angiography | 19.4% | 54.8% | <.001 |
| Days to coronary angiography ** | 15.0 (10.0–24.5) | 9.00 (5.0–13.0) | <.001 |
| Percutaneous intervention | 3.3% | 23.0% | <.001 |
| Days to percutaneous intervention ** | 21.0 (14.0–33.0) | 10.5 (5.0–17.0) | <.001 |
| Coronary artery bypass graft surgery | 5.5% | 8.4% | .054 |
| Days to coronary artery bypass graft surgery ** | 29.0 (22.5–51.0) | 23.0 (15.5–33.5) | .015 |
| Drug treatment at discharge | |||
| Calcium antagonists | 20.1% | 19.0% | .606 |
| Statins | 13.6% | 66.2% | <.001 |
| Angiotensin-converting enzyme inhibitors | 29.0% | 52.6% | <.001 |
| Beta-blockers | 32.4% | 57.1% | <.001 |
| Antiplatelet drugs | 86.7% | 91.7% | .002 |
| Nitrates | 30.9% | 22.9% | <.001 |
* Mean (standard deviation).
** Median (first quartile–third quartile) for patients undergoing the procedure.
The proportion of patients who died or developed post-infarction angina by 30 days was significantly lower in the second period. The proportion with complications remained at a similar level (Table 2).
Table 2. Proportion of Myocardial Infarction Patients Experiencing a Major Event Within 30 Days of Symptom Onset and Within 2 Years in 30-Day Survivors, by Period of Admission Without (1995–1997) and With (1999–2003) an On-Site Catheterization Laboratory.
| 1995–1997 | 1999–2003 | P-value | |
| Events within 30 days, n | 641 | 898 | |
| Cardiovascular death | 11.2% | 6.35% | .001 |
| Reinfarction | 3.25% | 3.64% | .697 |
| Post-myocardial infarction angina | 22.4% | 15.1% | <.001 |
| Killip class III–IV | 17.2% | 14.3% | .115 |
| Events within 2 years in 30-day survivors | 569 | 841 | |
| All-cause death | 12.3% | 7.61% | .003 |
| Cardiovascular death | 8.29% | 4.78% | .008 |
| Myocardial infarction | 6.59% | 7.81% | .475 |
| Unstable angina | 4.43% | 2.79% | .115 |
| Coronary catheterization | 4.21% | 8.10% | .014 |
| Percutaneous intervention | 1.55% | 4.65% | .008 |
| Coronary artery bypass graft surgery | 0.23% | 0.27% | 1 |
Killip class III–IV: acute pulmonary edema or cardiogenic shock.
Follow-up for mortality at 2 years was completed in all patients (the cause of death was unknown in 6, or 0.4%) who survived the acute phase for 30 days: 569 patients in the first time period and 841 in the second. In total, 499 patients (87.7%) in the first period were still alive after 2 years compared with 777 (92.4%) in the second. All-cause and CVD mortality 2 years after discharge was significantly lower in the second period (Table 2).
Follow-up for readmission due to MI or unstable angina was completed in 455 of the 499 surviving patients (91.2%) from the first period and in 755 of the 777 (97.2%) from the second period (Table 2).
Overall all-cause mortality from admission to 2 years was 22.2% and 13.5% in the first and second time periods, respectively (P<.001), and cardiovascular mortality was 18.6% and 10.9%, respectively (P<.001).
Table 3 shows the demographic, clinical and treatment characteristics and risk factors for patients who did or did not die of cardiovascular disease within 30 days for both time periods combined. Patients who experienced this type of event were older, were more frequently female, and were less frequently smokers or hypercholesterolemic. In addition, they received thrombolysis less often, were in a poorer Killip class, underwent more angiographic procedures, and more often had anterior MI and unclassifiable ECG findings.
Table 3. Demographic, Clinical and Treatment Characteristics and Risk Factors for Patients Who Did or Did Not Die of Cardiovascular Disease Within 30 Days, for the Two Time Periods Combined.
| Death | |||
| No | Yes | P-value | |
| Patients, n | 1410 | 129 | |
| Period 1999-2003 | 59.6% | 44.2% | .001 |
| Age, years * | 61.3 (11.4) | 69.6 (9.24) | <.001 |
| Percentage women | 20.1% | 31.0% | .004 |
| Risk factor history | |||
| Hypertension | 49.3% | 55.7% | .174 |
| Diabetes | 25.9% | 33.3% | .079 |
| Hypercholesterolemia | 48.8% | 32.2% | .001 |
| Current smokers | 42.0% | 21.7% | <.001 |
| ECG myocardial infarction findings | |||
| Non-Q-wave | 23.3% | 14.0% | |
| Inferior | 40.4% | 27.9% | |
| Anterior | 32.8% | 40.3% | |
| Unclassifiable | 3.6% | 17.8% | <.001 |
| Type of acute coronary syndrome | |||
| ST-elevation | 77.9% | 76.0% | |
| Non-ST-elevation | 18.4% | 5.4% | |
| Unclassifiable | 3.7% | 18.6% | <.001 |
| Thrombolysis | 42.1% | 25.2% | <.001 |
| Killip class III–IV | 11.0% | 68.1% | <.001 |
| Coronary angiography at admission | 42.7% | 10.5% | <.001 |
| Antiplatelet drugs at admission | 97.5% | 80.8% | <.001 |
Killip class III–IV: acute pulmonary edema or cardiogenic shock.
* Mean (standard deviation).
Table 4 shows the demographic, clinical and treatment characteristics and risk factors for MI patients who were still alive at 30 days and who did or did not die of cardiovascular disease within 2 years. Patients who experienced an event were significantly older, were more frequently female, diabetic or smokers, more often had unclassifiable ECG findings, and more often belonged to the second time period, although the difference for the last parameter was only marginally significant. They were also more likely to have received ACE inhibitors and less likely to have received beta-blockers or to have undergone thrombolysis or coronary angiography (i.e. during admission; they generally underwent many more angiographies after discharge than those still alive).
Table 4. Demographic, Clinical and Treatment Characteristics and Risk Factors for Myocardial Infarction Patients Who Were Alive at 30 Days and Who Did or Did Not Die of Cardiovascular Disease Within 2 Years, for the Two Time Periods Combined.
| Cardiovascular death | |||
| No | Yes | P-value * | |
| Patients, n | 1317 | 87 | |
| Period 1999–2003 | 60.5% | 46.0% | .008 |
| Age, years † | 60.7 (11.2) | 69.9 (9.52) | <.001 |
| Percentage women | 19.2% | 33.3% | .001 |
| Risk factor history | |||
| Hypertension | 48.8% | 54.1% | .317 |
| Diabetes | 24.5% | 47.7% | <.001 |
| Hypercholesterolemia | 48.8% | 49.4% | .914 |
| Current smokers | 43.3% | 22.6% | <.001 |
| ECG myocardial infarction findings | |||
| Non-Q-wave | 23.1% | 26.4% | Reference |
| Inferior | 40.9% | 31.0% | .155 |
| Anterior | 33.0% | 31.0% | .569 |
| Unclassifiable | 3.0% | 11.5% | .002 |
| Type of acute coronary syndrome | |||
| ST-elevation | 78.2% | 73.6% | |
| Non-ST-elevation | 18.6% | 14.9% | |
| Unclassifiable | 3.2% | 11.5% | <.001 |
| Thrombolysis | 43.9% | 16.3% | <.001 |
| Killip class III–IV | 9.0% | 41.9% | <.001 |
| Treatment prescribed at discharge | |||
| Statins | 49.8% | 27.8% | <.001 |
| Angiotensin-converting enzyme inhibitors | 45.0% | 57.0% | .030 |
| Beta-blockers | 53.1% | 19.0% | <.001 |
| Antiplatelet drugs | 94.3% | 89.3% | .062 |
| Coronary angiography within initial 30 days | 43.8% | 25.6% | .001 |
| Coronary angiography within 30 days to 2 years | 6.0% | 15.4% | <.001 |
Killip class III–IV: acute pulmonary edema or cardiogenic shock.
* Estimated by Cox regression analysis.
† Mean (standard deviation).
Table 5 shows the characteristics of patients who did or did not undergo coronary angiography, regardless of PCI, in the two study periods. The use of coronary angiography in patients with non-ST-elevation ACS was higher in the second period.
Table 5. Characteristics of Patients Who Did or Did Not Undergo Coronary Angiography in the Two Study Periods, Irrespective of Percutaneous Intervention.
| 1995–1997 | 1999–2003 | ||||||
| Coronary angiography | Coronary angiography | ||||||
| No | Yes | P-value a | No | Yes | P-value b | P-value c | |
| Patients, n | 508 | 122 | 400 | 485 | |||
| Age, years d | 64.3 (12.2) | 61.9 (10.2) | .052 | 61.2 (11.2) | 60.3 (10.7) | .212 | .116 |
| Percentage women | 22.0% | 21.3% | .86 | 17.0% | 22.7% | .036 | .746 |
| Risk factor history | |||||||
| Hypertension | 45.5% | 50.0% | .372 | 46.9% | 57.1% | .003 | .158 |
| Diabetes | 26.5% | 29.2% | .548 | 25.1% | 26.7% | .585 | .589 |
| Hypercholesterolemia | 40.7% | 43.0% | .645 | 47.7% | 55.8% | .018 | .012 |
| Current smokers | 37.1% | 32.0% | .289 | 46.3% | 41.1% | .132 | .064 |
| ECG myocardial infarction findings | |||||||
| Non-Q-wave | 15.9% | 18.9% | 18.0% | 34.6% | |||
| Inferior | 44.3% | 37.7% | 47.2% | 28.7% | |||
| Anterior | 34.1% | 41.8% | 31.0% | 32.2% | |||
| Unclassifiable | 5.71% | 1.64% | .091 | 3.75% | 4.54% | <.001 | .001 |
| Type of acute coronary syndrome | |||||||
| ST-elevation | 82.1% | 83.6% | 81.8% | 68.5% | |||
| Non-ST-elevation | 12.0% | 14.8% | 14.5% | 26.6% | |||
| Unclassifiable | 5.91% | 1.64% | .126 | 3.75% | 4.95% | <.001 | .004 |
| Thrombolysis | 38.7% | 50.8% | .014 | 45.6% | 35.4% | .002 | .002 |
| Killip class III–IV | 17.6% | 15.6% | .601 | 14.1% | 14.2% | .991 | .693 |
Killip class III–IV: acute pulmonary edema or cardiogenic shock.
Note: data on coronary angiography were not available for 11 patients in the first period and 13 in the second.
a no coronary angiography versus coronary angiography for the first time period
b no coronary angiography versus coronary angiography for the second time period
c no coronary angiography versus coronary angiography for both time periods combined
d mean (standard deviation)
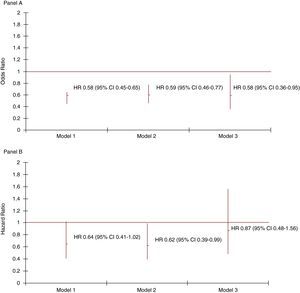
Figure 1 shows the ORs for death at 30-day follow-up for the second time period as the number of factors for which adjustment was made in the logistic models increased (Figure 1, Panel A). The risk of death in this period was significantly lower, regardless of the level of adjustment. The Cox model showed that the HR for cardiovascular death at 2-year follow-up in 30-day survivors was significantly lower in the second period (Figure 1, Panel B) after adjustment for age, sex, hypertension, dyslipidemia, smoking, diabetes, ECG MI findings, acute pulmonary edema or cardiogenic shock, and thrombolysis (Figure 1, Panel B, model 2). However, this effect was confounded by statins, ACE inhibitors, beta-blockers and antiplatelet drugs being prescribed at discharge (Figure 1, Panel B, model 3). The HR for all-cause death among 30-day survivors was 0.73 (95% confidence interval [CI] 0.50–1.06), 0.72 (95% CI 0.50–1.05) and 0.89 (95% CI 0.56–1.42) for the second period for the three models adjusted as indicated in Figure 1, Panel B.
Figure 1. Adjusted hazard ratio (HR) for death at 30 days (Panel A) and for cardiovascular death at 2 years in 30-day survivors (Panel B) in the period of hospital admission with an on-site catheterization laboratory (i.e. 1999–2003) compared with the period without (i.e. 1995–1997). CI: confidence interval; HR: hazard ratio.Panel AModel 1: adjusted for age, sex, hypertension, dyslipidemia, smoking, diabetes and ECG myocardial infarction findings.Model 2: adjusted as for Model 1 with the addition of acute pulmonary edema and cardiogenic shock.Model 3: adjusted as for Model 2 with the addition of thrombolysis and antiplatelet drug use on admission.Panel BModel 1 adjusted for age, sex, hypertension, dyslipidemia, smoking, diabetes, ECG myocardial infarction findings, acute pulmonary edema and cardiogenic shock.Model 2: adjusted as for Model 1 with the addition of thrombolysis.Model 3: adjusted as for Model 2 with the addition of the prescription of statins, angiotensin-converting enzyme inhibitors, beta-blockers and antiplatelet drugs at discharge.
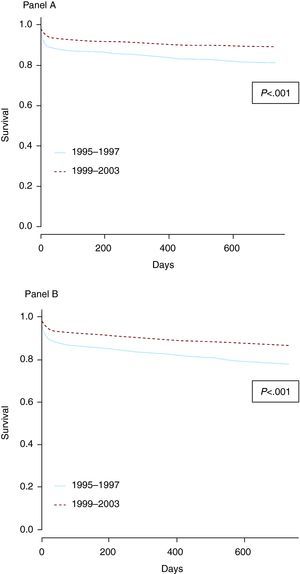
Figure 2 shows survival curves from admission to 2 years for the two time periods: for cardiovascular death in Panel A and for all-cause death in Panel B. In both cases, the survival rate was significantly better at 2 years for the 1999–2003 time period, when a catheterization laboratory was available on site.
Figure 2. Survival curves for death from coronary heart disease (Panel A) and all causes (Panel B) from admission to 2 years, for both time periods.
DiscussionThe results of this 2-year follow-up cohort study of patients admitted to the same tertiary hospital during two distinct time periods, without and with a diagnostic catheterization laboratory on site, show that the availability of the facility resulted in the increased use of invasive strategies, a shorter delay before treatment, and lower 30-day mortality, which persisted at 2 years. The improvement observed at 2 years principally stemmed from the results obtained within the initial 30 days following admission, combined with the increased prescription of statins, ACE inhibitors, beta-blockers and antiplatelet drugs at discharge. The shorter delay before treatment and increased use of all the diagnostic and therapeutic procedures made available by the catheterization laboratory indicate that the overall management of MI patients during the acute phase may well be the reason for the observed benefit.
Although the easy availability of invasive cardiac care facilities is associated with an increase in their use, their influence on outcomes is not clear, especially when a simultaneous increase in the use of drug treatment is taken into account.17,18,19,20 The RESCATE study compared treatment and outcomes over the same time period in a number of Spanish hospitals with and without on-site catheterization laboratories and found that those with a catheterization laboratory performed more procedures, with a shorter treatment delay. However, no difference in mortality was found at 6 months.5 Another study compared treatment and outcomes during the same period at two hospitals with markedly different rates of catheterization use and found that survival at 28 days was significantly better in the hospital with higher use.6 A study of the records of 19 hospitals in Seattle found no difference in mortality despite differences in the rates of use of coronary angiography and coronary angioplasty.17 In a French study, the lower mortality associated with admission to a hospital with on-site angioplasty facilities was attributed to greater use of evidence-based medical treatment.21 A post hoc analysis of the Gusto-IIb trial database showed that, despite a higher rate of interventions in the United States compared with Canada, 1-year mortality was comparable.22 Most of these studies date from the time before the widespread use of primary angioplasty.
It is known that the use of primary angioplasty was relatively low in Spain during the period studied. The figures reported in our study are consistent with those recorded by Spanish national registries early in the decade.23,24
Study Advantages and Potential LimitationsOne particularly positive feature of this study was that the evaluation was carried out at a single hospital, thereby avoiding any variability in patient management between centers.
Although the on-site catheterization laboratory first became operational in 1998, initially only diagnostic coronary angiography was carried out. Patients were referred to other tertiary hospitals for angioplasty procedures. This enabled us to evaluate the contribution the catheterization laboratory made to the reduction in the 2-year event rate independent of the use of angioplasty, despite the fact that the total number of procedures carried out in our case may have been lower than that recommended by current criteria.
In our study, we took into account the treatment prescribed at discharge (this treatment is practically free for the general population in Spain), but we had no information on compliance or on any side effects during the 2-year follow-up period. This may have introduced some unknown biases that would clearly not have operated in favor of the study hypothesis because currently available data suggest that the medication compliance of MI patients has not changed in the past 10 years.25
One limitation of the study is that it did not consider drug use at admission. We observed an increase in the use of beta-blockers (from 30% in 1993 to 83% in 2003) and ACE inhibitors (from 36% in 1996 to 74% in 2003). The use of thrombolysis (45% in 1993 and 37% in 2003) and platelet inhibitors (94% in 1993 and 98% in 2003) has remained stable over the past 15 years, according to REGICOR data (unpublished findings). The Spanish MI registry also showed a decrease in 28-day mortality (from 14.2% to 11.3%; P<.001) and 1-year mortality (from 18.5% to 16.4%; P<.001) between 1995 and 2000 which was associated with the increased use of both reperfusion techniques and medical treatment.26 Consequently, some undetermined part of the beneficial effect of the “time period” on 30-day mortality might have been due to these changes.
Moreover, ejection fraction was not taken into account because it was not documented systematically during the first time period.
The increase observed in the prevalence of hypertension, diabetes, and hypercholesterolemia may have been due to changes in diagnostic criteria that occurred at the end of 1990s.27,28,29
The variation in treatment received by these patients, which resulted from the different treatment guidelines in force during the two time periods, could have resulted in some variation in the use of coronary angiography.
Finally, our analysis of the effect of coronary angiography and PCI was limited by the difference in survival during the acute phase (i.e. in the first few hours after symptom onset). Therefore, the models were not adjusted for these variables because that could have led to their actual effect being overestimated due to reverse causality. The hypothesis under analysis was restricted to the effect of the availability of a catheterization laboratory (i.e. the “time period” effect).
ConclusionsThe opening of a new catheterization unit in a tertiary hospital was associated not only with an increase in the number of coronary catheterizations and PCIs carried out in MI patients, but also with a reduction in the time delay before these procedures were implemented.
The greater availability of this facility increased its use and broadened the range of indications for referral.
The result was lower cardiovascular mortality at 30 days, which was maintained at 2 years. Lower mortality persisted after hospital discharge and was combined with the effect of more intensive secondary prevention with statins, ACE inhibitors, beta-blockers and antiplatelet drugs, which were prescribed at discharge.
FundingThis project was supported by grants from the Agència d’Avaluació de Tecnologia i Recerca Mèdiques (069/18/02), by the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III/ FEDER (Red HERACLES RD06/0009), and by the Agència de Gestió d’Ajuts Universitaris I de Recerca, Generalitat de Catalunya (2009SGR1195).
Conflicts of interestNone declared.
Acknowledgements
The authors wish to acknowledge the contribution of the Catalan Department of Health's death certificate registry and to thank Susana Tello and Lenny Franco for data management, Izabella Rohlfs for organizing data collection, Ruth Martí, Isabel Ramió, Sergi Moral and Xavier Oliva for collecting the data, and Elaine Lilly, Ph.D., for English language editing.
Received 22 January 2010
Accepted 9 July 2010
Corresponding author: Programa de Investigación en Procesos Inflamatorios y Cardiovasculares (RICAD), Institut Municipal d’Investigació Mèdica (IMIM), Dr. Aiguader, 88, 08003 Barcelona, Spain. jmarrugat@imim.es