Although congenital heart defects are the most common major congenital abnormalities, the associated mortality has been decreasing due to improvements in their diagnosis and treatment. We assessed the usefulness of 64-multidetector computed tomography in the diagnosis and management of these patients.
MethodsThis 5-year observational, analytical, retrospective, cohort study included a total of 222 tomographic studies of patients with congenital heart disease. Computed tomography scans were read twice and medical records were reviewed. We assessed the complexity of the disease, patient, and radiological technique, and evaluated the contribution of new data in relation to clinical suspicion and diagnostic change. A confidence interval was set at 95% and a P value of<.05 was used as the cutoff for statistical significance.
ResultsIn 35.1% of patients, the treatment procedure was performed after computed tomography without other tests. Additional diagnostic catheterization was performed in 12.5% of patients. There were new findings in 77% of patients (82.9% with complex disease), which prompted a change in patient management in 35.6%. All unexpected reports described new findings. No significant differences were found by age, sex, study period, urgency of the test order, patient complexity, or difficulty of the technique.
ConclusionsUse of 64-detector computed tomography yields good diagnostic performance in congenital heart disease, prompts changes in management in more than one-third of patients, and reveals new findings in relation to the presumed diagnosis in 77% of patients.
Keywords
Congenital heart disease (CHD) is defined as any structural heart defect with real or potential significance. Its incidence ranges between 4/1000 live births and 12/1000 live births and the most common CHDs are major congenital defects. Environmental factors, isolated chromosomal abnormalities, or chromosomal abnormalities associated with a set of syndromes are the most common known causes. The majority of CHDs are due to abnormalities that develop between 3 and 10 weeks of gestation and are dynamic entities that have a different course in each patient. There is a progressive decrease in mortality (better diagnosis and treatment) and thus an increase in survival (approximately 85%-90% of CHD patients reach adulthood).1
Although it is difficult to determine the influence of imaging techniques on the increase in survival, there has been a demonstrated increase in the detection of CHD following the introduction of 64-slice multidetector computed tomography (64-MDCT) and the widespread use of cardiac magnetic resonance imaging (MRI). The diagnostic information obtained using low-risk techniques is essential for optimal medical and surgical management2–4 and perioperative imaging is useful in assessing procedural success.5,6
Continuing advances in medical imaging have made it possible to diagnose cardiovascular disease in any patient by the use of different methods involving a wide variety of technical requirements, benefits, limitations, and costs. The correct application of each method requires a well-integrated group of experts who collaborate with the clinical diagnostic services.7,8 There is a trend toward the decreased use of diagnostic catheterization despite its being the reference diagnostic technique and its use is now mainly reserved for therapeutic decision-making.9
We are conducting a multidisciplinary study on managing CHD in our hospital. The incorporation of 64-MDCT led us to reflect on the capacity of this new technique to provide answers to the clinical problems of CHD patients and evaluate its implications for their subsequent management. This article assesses the impact of the introduction of 64-MDCT on the management of CHD patients.
As far as we know, this is the first study to analyze the clinical application of 64-MDCT in a large cohort of mainly pediatric CHD patients, in relation to the reports submitted to clinicians, and to determine the role, accuracy, and safety of this technique for this group of patients.
METHODSStudy Design and PopulationThe study followed an observational retrospective cohort design. All patients were seen by a radiologist with more than 10 years’ experience in the diagnosis of CHD using MRI and cardiac computed tomography (CT) at the Radiology Department of the Hospital La Paz, Madrid. This hospital is a national tertiary referral center for pediatric and adult CHD. The duration of the study was 5 years (January 2006 to January 2011).
We coded all patients with an ordinal number and examination number to comply with current Spanish regulations on the protection of personal data (Law 15/1999, issued on March 5, 2011). In all cases, written consent was obtained from the patient or guardian. The study was approved by the clinical research ethics committee of the hospital (PI-1260).
All patients had a previous transthoracic echocardiogram. Patients fulfilling the following criteria were included: suspected noncoronary CHD (previously known or not); suspected noncoronary CHD needing follow-up or posttreatment follow-up (surgical or endovascular); CHD and suspected coronary artery disease, and pediatric patients with suspected pulmonary hypertension with or without known heart disease. We excluded follow-up data that did not provide information on indications or diagnosis.
Study ProtocolWe used an Aquillion 64 CT scanner, model V6.2ER014 (Toshiba Medical Systems Europe). Age, suspected disease, and patient collaboration guided the radiological technique used (simple contrast-enhanced CT, CT angiography, or cardiac CT) and the need for anesthesia (age<6 years).
Contrast agent (iodine, 300mg/mL) was intravenously administered to all patients at 2mL/kg body weight using an automatic dual-head CT contrast delivery system. The injection rate was adjusted according to the vessel cannulated. All patients fasted for 4h (neonates) or for 6h.
We performed simple CT and CT angiography during free breathing and acquired a single spiral image from the thoracic outlet to the dome of the diaphragm. However, in the case of an extracardiac Fontan procedure images were obtained during late acquisition (70s) (Figure 1). Acquisition was started between 35s and 40s after the administration of intravenous contrast agent during simple CT and with automatic bolus tracking during CT angiography. Both techniques were performed using automatic tube current modulation, rotation 0.5s, 1.0 pitch to 1.5 pitch, thin collimation 0.5mm×64mm, and slice thickness 0.5mm.
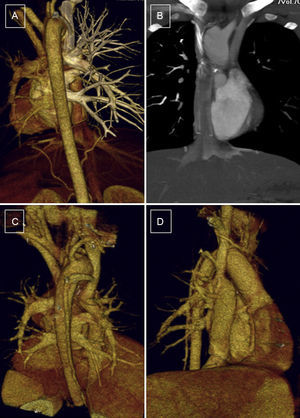
Patient, 10 years old, with bilateral bidirectional Glenn shunt and total cavopulmonary connection. Complete filling of the ducts without evidence of thrombus or stenosis in the surgical connections. A and B: arterial phase, without contrast filling, left Glenn procedure and Fontan procedure. C and D: venous phase, homogenously filled ducts, posterior view and anterior right view.
Cardiac CT was performed during apnea (5-15s) with automatic bolus tracking, 0.2 pitch to 0.3 pitch (P=.206), thin collimation (64mm×0.5mm), slice thickness 0.5mm, high kilovoltage and milliamperage, retrospective electrocardiographic synchronization, and reconstructions with 30% to 50% slice overlap.
Images were reconstructed using the aorta, cardio, and airways presets of the Vitrea® 2 console (Vital Images, Inc.; MediMark® Europe). Three-dimensional volumetric reconstruction, multiplanar reconstruction (MIP [maximum intensity projection], MiniIP [minimum intensity projection]), and vascular measurements were obtained by sequential segmental analysis. Images were obtained from the image archive of the hospital for the second reading and the 64-MDCT imaging study reports and clinical histories were reviewed for developments subsequent to 64-MDCT.
Variables CollectedPopulation data: age, sex, date of test. Clinical information contained in the test order: purpose and justification of the test and pre- or posttreatment study. Clinical history: additional pre- and post-64 MDCT tests. The complexity of heart disease10,11 and of each patient was calculated (Aristotle score).12,13 Studies were classified by their technical difficulty: simple chest CT with intravenous contrast, CT angiography, and adult and pediatric cardiac CT. We assessed the need for using cardiac synchronism.
Anatomical findings were coded by group: aortic disease, pulmonary tree, heart disease, anomalous pulmonary venous drainage, complex CHD, postprocedural complications, pulmonary hypertension, airways, and other (unexpected findings).
We assessed whether the findings prompted a change in clinical management, treatment, or patient lifestyle (scored as 1, known findings; 2, new findings without change in management; 3, new findings with a change in management; 4, change in diagnosis). We also assessed concordance between the presumptive diagnosis and the diagnostic test: existence of expected or unexpected findings.
New findings were defined as any anatomical abnormality not found in previous tests (transthoracic echocardiography, transesophageal echocardiography, MRI, etc):
- •
A change in diagnosis: changes in CHD complexity, chamber sequence, or in previously diagnosed intracardiac or great vessel defects:
- -
Changes in treatment: from surgery to catheterization, from catheterization to surgery, or changes in the catheterization or surgical technique used.
- -
No changes in treatment.
- -
- •
No changes in diagnosis.
Unexpected findings were defined as findings that were not predicted by the known natural history of the main anatomical abnormality or changes in its natural history.
Statistical AnalysisThe analytical study (SPSS version 11.5 for Windows) was conducted in collaboration with the Biostatistics Department of the Hospital La Paz. The confidence interval was set at 95% and a P value<.05 was used as the cutoff for statistical significance.
The Student t test or analysis of variance were used to compare continuous quantitative variables with a normal distribution and nonparametric tests otherwise (Mann-Whitney U test or Kruskal-Wallis test). The Bonferroni post-hoc test was applied when more than 2 groups were compared using analysis of variance. Qualitative variables were compared using the Pearson chi-square test or Fisher exact test.
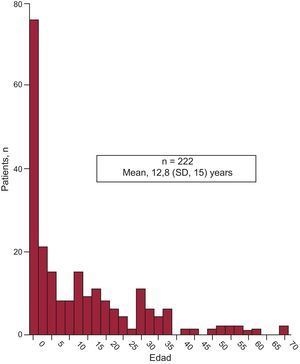
RESULTSIn total, 222 patients were included. The population was asymmetric (136 men and 86 women) with a mean age of 12.8 years (1 day-71 years) (Figure 2).
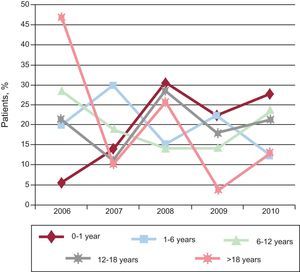
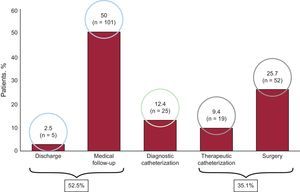
In 2006, the oldest age group consisted of patients > 18 years (47.5%), but the size of this group decreased over time. The only patient group that increased in size was aged 0-year to 1 year. The size of the other groups remained steady over time (Figure 3). In total, 71.2% of patients did not undergo further studies except for transthoracic echocardiography. Tables 1 and 2 show the main diagnoses. A total of 87.6% patients did not need further diagnostic tests because they were either discharged (2.5%) or followed up in outpatient visits (50%). The therapeutic procedure was performed without additional tests in 35.1% of patients. Additional catheterization was performed in only 12.5% of patients (Figure 4). Of the patients studied, 90% had moderate to severe CHD and 80.2% had a level of complexity <3 (level I).
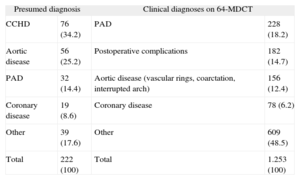
List of Main Diagnoses Recorded
| Presumed diagnosis | Clinical diagnoses on 64-MDCT | ||
| CCHD | 76 (34.2) | PAD | 228 (18.2) |
| Aortic disease | 56 (25.2) | Postoperative complications | 182 (14.7) |
| PAD | 32 (14.4) | Aortic disease (vascular rings, coarctation, interrupted arch) | 156 (12.4) |
| Coronary disease | 19 (8.6) | Coronary disease | 78 (6.2) |
| Other | 39 (17.6) | Other | 609 (48.5) |
| Total | 222 (100) | Total | 1.253 (100) |
64-MDCT, 64-detector computed tomography; CCHD, complex congenital heart disease; PAD, pulmonary artery disease.
The left column shows the most common presumed diagnoses (indications for testing). The right column shows the diagnoses after performing and analyzing the 64-slice multidetector computed tomography studies.
Data are expressed as No. (%).
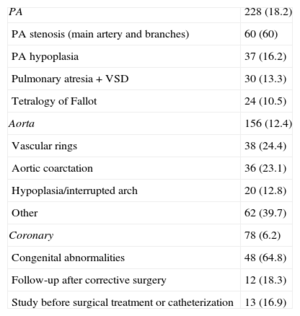
Most Common Specific Diagnoses on 64-Multidetector Computed Tomography
| PA | 228 (18.2) |
| PA stenosis (main artery and branches) | 60 (60) |
| PA hypoplasia | 37 (16.2) |
| Pulmonary atresia+VSD | 30 (13.3) |
| Tetralogy of Fallot | 24 (10.5) |
| Aorta | 156 (12.4) |
| Vascular rings | 38 (24.4) |
| Aortic coarctation | 36 (23.1) |
| Hypoplasia/interrupted arch | 20 (12.8) |
| Other | 62 (39.7) |
| Coronary | 78 (6.2) |
| Congenital abnormalities | 48 (64.8) |
| Follow-up after corrective surgery | 12 (18.3) |
| Study before surgical treatment or catheterization | 13 (16.9) |
PA, pulmonary artery; VSD, ventricular septal defect.
Postoperative complications were varied with a predominance of systemic-pulmonary collaterals.
Data are expressed as No. (%).
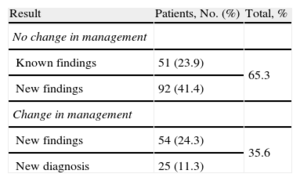
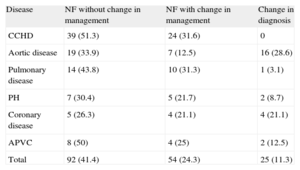
New findings were observed in 77% of patients. Mutually exclusive dichotomous categories were as follows: no change in management (65.3%); subsequent change in management (35.6%), of which 11.3% were due to changes in diagnosis (Table 3). Regarding disease, 82.9% of new findings were complex CHD, with changes in treatment in 31.6% (P<.0001), and changes in diagnosis in aortic disease (28.6%) and coronary artery disease (21.1%). There were no changes in the diagnosis of complex CHD (Table 4). Regarding CHD complexity, there were new findings in 81.8% patients with severe CHD, with changes in management in 31.2%. There were changes in diagnosis in patients with moderate CHD (18.9%; P<.05) and changes in diagnosis in patients with greater complexity (mean score 2.72; P<.05). Simple CT pediatric studies contributed to clinically significant new findings in 66.7% of patients (P<.05). Pediatric cardio-CT contributed to new findings in 80% of patients and led to subsequent changes in management in 30% (P<.05). All unexpected reports (100%) described new findings prompting changes in management in 86.8% of patients and a new diagnosis in 47.2% (P<.0001). There was a change in management in only 19.5% of the patients with an expected report.
New Findings by Disease
| Disease | NF without change in management | NF with change in management | Change in diagnosis |
| CCHD | 39 (51.3) | 24 (31.6) | 0 |
| Aortic disease | 19 (33.9) | 7 (12.5) | 16 (28.6) |
| Pulmonary disease | 14 (43.8) | 10 (31.3) | 1 (3.1) |
| PH | 7 (30.4) | 5 (21.7) | 2 (8.7) |
| Coronary disease | 5 (26.3) | 4 (21.1) | 4 (21.1) |
| APVC | 8 (50) | 4 (25) | 2 (12.5) |
| Total | 92 (41.4) | 54 (24.3) | 25 (11.3) |
APVC, anomalous pulmonary venous connection; CCHD, complex congenital heart disease; NF, new findings; PH, pulmonary hypertension.
Data are expressed as No. (%).
Regarding the match between presumptive diagnosis and diagnosis after CT testing, the highest percentage of unexpected reports was reached in 2006 (37.7%; P<.05) with a predominance of aortic disease (37.5%) and coronary artery disease (31.6%; P<.05), whereas in 2009 there was a predominance of aortic disease (56.3%; P<.05).
Urgent test orders were a significant factor (P<.005) and there was an unexpected report in 39.3% of these patients vs 14.6% and 20.3% of patients with scheduled or standard orders, respectively. Regarding urgent orders, more unexpected reports were observed in patients with aortic disease and in patients<1 year old (P<.01). Significant differences were found in unexpected reports of moderately complex heart disease (30.3%; P<.05) and in patients <1 year old compared to other age groups (P<.05). Patient complexity scores were higher in studies with an unexpected report (mean, 2.3; P<.01) and in patients <1 year old (mean, 3.12; P<.0001). When 2×2 comparisons were made with the nonparametric test, significant differences were found in patients <1 year old. There were significant differences between 2006 and the other periods (P<.05).
Patient complexity was greater in patients with pulmonary hypertension (3.17 points), although no differences were found in group means. In total, 66.7% of the unexpected reports were found by pediatric simple CT (P<.05), principally in patients with aortic disease (P<.01).
There was no significant association between sex and an expected/unexpected report, but confounding factors (35.7% vs 44.3%) due to chance may have been present (independent factors associated with the diagnosis).
DISCUSSIONAlthough MDCT-64 provides excellent anatomic imaging quality compared to MRI, it has the great disadvantage of using ionizing radiation. Many publications have discussed the diagnostic value of 64-MDCT in CHD, mainly first- and second-level studies (diagnosis and technical parameters).14 To our knowledge, very few studies have investigated the impact of 64-MDCT on the therapeutic approach and management of these patients.15 Ideally, the study should follow a randomized controlled design, but these studies would have been long, expensive, and unethical. Therefore, we performed a retrospective study by following up clinical expert opinions based on 64-MDCT reports.
We observed that nearly half of the data collected in the later period were new findings (due to more experience and more accurate techniques and diagnosis). If these new findings had just led to new diagnoses, this situation could have been explained by a lack of expertise or wrong diagnosis on the part of clinicians. However, our results suggest greater diagnostic accuracy. Changes in management were more frequent in younger patients. Regarding all unexpected findings, there was a change in management in 86.8% of patients. In an MRI study, Secchi et al16 found that unexpected reports were associated with more clinically important categories and 85% of unexpected reports prompted a change in treatment, lifestyle, or diagnosis. No significant differences were found between study periods, indicating that clinicians used consistent criteria when interpreting the test reports. Tsai-Goodman et al3 found that 70.3% of patients who underwent MRI did not require additional catheterization. In our study, 78.83% of patients did not require additional diagnostic studies. Therefore, the values obtained are better and provide sufficient information for decision making. The technique used is not invasive and avoids additional costs and risks. Eichhorn et al17 also found that multidetector CT has high diagnostic accuracy, avoiding the need for additional techniques to plan the surgical approach. According to these authors, the diagnostic accuracy of CT is comparable to catheterization and is more accurate in detecting other complications that could put the patient's life at risk. Khatri et al18 suggested that 64-MDCT has many advantages over other conventional diagnostic techniques and that it provides key data concerning the surgical and interventional approach to adopt. These authors found excellent correlation between 64-MDCT images and surgical findings and suggested that 64-MDCT should be considered the leading diagnostic technique in CHD and the best technique on which to base decisions. Their study was the first to suggest that MDCT-64 could replace catheterization as a noninvasive diagnostic technique in CHD.
Juan et al19 suggested that although 64-MDCT is good diagnostic technique in CHD, because of its lack of a therapeutic role it cannot replace catheterization. We performed additional diagnostic catheterization in only 21.17% of patients, the majority for therapeutic objectives or as additional functional studies. Lee et al20 found that additional diagnostic cardiac catheterization was not needed in a group of neonates following 64-MDCT. We agree with the authors that after initial assessment with transthoracic echocardiography, 64-MDCT could probably replace diagnostic catheterization, particularly in neonates, given that the rate of catheterization-associated complications still ranges from 10% to 20% and catheterization-associated mortality is around 1%. Vastel-Amzallag et al21 also suggested in their study on tetralogy of Fallot that 64-MDCT avoids additional angiographic analysis before corrective surgery. These changes in diagnostic approach have also been addressed in recent publications and guidelines on interventional cardiology.22
We assessed the correlation between the presumptive and definitive diagnosis according to the percentage of unexpected results and diagnostic changes. Unexpected reports described new findings in 100% of patients and prompted changes in management in 86.8% of patients. Of these, 47.2% were new diagnoses. There was a change in management in only 19.5% of the patients with an expected report. Secchi et al16 found that cardiac magnetic resonance reports were judged as unexpected in only 13% of patients, prompted no change in treatment in 70%, and prompted a change in diagnosis in 5%. Overall, we obtained better results. There were no significant differences by age group and expected/unexpected report, suggesting that a diagnosis of CHD is independent of age.
We also found no differences in the reason for the study (pre- or post-surgical intervention or pre- or post-catheterization) and the purpose of the test (for assessment or monitoring, or to confirm or reject diagnosis). For ethical reasons, we ensured that the performance and interpretation of 64-MDCT did not effect the wellbeing of the patients. In 2009, we observed more unexpected reports, the majority of which described aortic disease needing urgent treatment23 (Figure 5). On the other hand, in complex CHD the percentage of unexpected reports was very low, suggesting that the diagnosis had been previously categorized using other techniques. Greater complexity was found in patients with unexpected reports and in younger patients in each age group (2×2, nonparametric test) (Figure 6). The clinical value of 64-MDCT can be appreciated by considering what would have happened to patients with unexpected findings. The diagnosis would probably have been reached by using other diagnostic techniques (MRI, catheterization) or by the natural course of the disease. However, this conclusion is speculative because the study did not include a control group.24
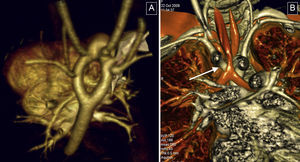
Vascular rings. These were the most common condition in the study. A: three-dimensional volumetric reconstruction, superior-posterior view; complete double aortic arch, with symmetrical arches and isolated origin in supraaortic trunks. B: reconstruction of the airways; reduced tracheal lumen at its passage through the vascular ring (arrow).
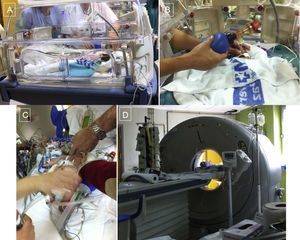
Procedure in a neonate with highly complex congenital heart disease. A: manual ventilation in transport cot. B: preparation before placement on 64-detector computed tomography table. C: situation on the table during final check of all the patient's leads and medication tubes. D: patient inside the image acquisition device.
In the setting of CHD, 64-MDCT has advantages compared to MRI: the simultaneous assessment of cardiovascular abnormalities via airways, lung parenchyma, and chest wall; short anesthesia times; greater spatial resolution; greater availability; less sensitivity to metal artifacts; less experience is required; it can be performed in patients with pacemakers or claustrophobia, and MRI cannot yet be used to study coronary arteries.6,25,26
Based on existing guidelines24,27–30 and our own experience, we summarize the indications for 64-MDCT in CHD (adults and children) into 5 main groups:
- 1.
Test ordered to confirm/reject anomalous origin or trajectory of proximal and distal coronary arteries.
- 2.
MRI contraindicated, large metal appliances, or major vascular calcifications.
- 3.
Assessment via airway, lung parenchyma, or chest wall (repeat surgeries).
- 4.
Abnormalities with tortuous vessels or low-diameter vessels.
- 5.
Patients in a severe clinical situation or in a situation that is difficult to control.
Although the damage caused by ionizing radiation can lead to cancer and is directly associated with the patient's age, no consistent cause-effect relationship has been found between the incidence of tumors and exposure to radiation for diagnostic purposes.31–33 There is no absolute contraindication to cardiovascular MDCT except iodine contrast allergy or contrast-induced nephrotoxicity. Once the MDCT study has been approved, the aim is to minimize the radiation dose needed to acquire an image of sufficient quality to obtain a correct diagnosis.34
Study LimitationsThe retrospective design of the study was ideal for post hoc statistical analysis. However, we based the definition of predicted data on speculations derived from the clinical information contained in the test order because otherwise it would have been more difficult to make predictions. Cases of CHD in patients attending a tertiary hospital are complex and may have been a source of selection bias, possibly leading to an overestimation in the results. The large number of neonates and infants in our study is explained by the fact that our hospital has a neonatal unit. Interobserver variability was not assessed.
CONCLUSIONSThis study shows that 64-MDCT has good diagnostic performance in CHD patients. The use of this technique has an impact on patient progress, prompts a change in patient management in more than a third of patients, and reveals new findings in relation to initial suspicion in 77% of patients. All unexpected reports (100%) described new findings that prompted changes in management in 86.8% of patients. Although it would be ideal to conduct more studies of this kind, we consider that the current rapid developments in technology make it difficult to conduct future comparative studies or studies involving other centers.
CONFLICTS OF INTERESTNone declared.