Keywords
INTRODUCTION
The relationship between exercise and its health benefits is well known. Nevertheless, the lack of physical activity continues to be a public health problem. The reduction in physical work has compromised physical fitness, and this change toward a more sedentary lifestyle is detrimental to the individual and potentially costly for society, as it has been accompanied by a simultaneous increase in the number of cases of cardiovascular disease. Humans, like animals, reduce their physical activity as they age and, in modern society, the level of voluntary physical activity declines as soon as maturity is reached. If the importance of exercise in the maintenance of cardiovascular and muscle health is taken into account, it is not surprising that the functional capacity and tolerance for physical exertion deteriorates in inactive adults.
On the other hand, there is sufficient information in the medical literature demonstrating the beneficial effects of physical activity on health and longevity.1 When exercise forms part of our occupational and recreational activities, it is beneficial in terms of preventing ischemic heart disease, decreasing overall mortality and improving quality of life. Thus, exercise plays an important role in the prevention of numerous conditions and is of great utility in delaying the negative effects of aging on the cardiovascular system.
PHYSICAL EXERCISE AND SPORTS
Physical activity and exercise are not interchangeable terms, although, with certain frequency, they are employed without distinction, and physical exercise and sports are even utilized as synonyms. Physical activity is any body movement produced by the skeletal muscles that results in energy expenditure. Physical exercise is a different concept, since it is a type of physical activity that is planned, structured and repetitive, and is performed for the purpose of maintaining or improving one or more fitness components. Physical aptitude or fitness is understood to be the capacity to perform mild to moderate physical activity without experiencing excessive fatigue. The concept of physical fitness includes different variables of cardiovascular fitness, respiratory fitness, body composition, muscle strength and elasticity, and flexibility.1 Sports involve organized matches, requiring physical exertion, that abide by an established structure and are coordinated within a context of formal and explicit rules and regulations with respect to behaviors and procedures. Sports are physical and intellectual activities that have a competitive component that involves spectacle and physical training.
Sports differ widely from one to another, as do the factors that define each specialty.2 On the basis of their bioenergetic characteristics, sports are classified as: a) aerobic, in which exercises of long duration and mild to moderate intensity predominate and in which oxygen delivery is fundamental in order to obtain energy (for example, a marathon); b) alactic anaerobic, which are exercises of short duration and very high intensity, in which the energy supply is provided by adenosine triphosphate (ATP) and phosphocreatine (for example, 50 and 60-meter track events); c) lactic anaerobic, involving exercises of short duration and high intensity (for example, 400-meter track events); and d) mixed, that is, aerobic-anaerobic (for example, soccer, basketball, and volleyball). Thus, the comparison of the effects of different sports can prove to be truly difficult, since the metabolic changes vary according to the predominant energy pathway, which, in turn, can differ depending on whether we are talking about training or a competition.3
TYPES OF PHYSICAL EXERCISE
The contractile phenomenon is a process that requires energy, and ATP is the only immediate source of energy for muscle contraction. Skeletal muscle utilizes 3 sources of energy for its contraction: the alactic anaerobic system (involved in high-intensity activities that take less than 15 to 30 seconds), the lactic anaerobic system or anaerobic glycolysis (exercises of maximum intensity and a duration of 30 to 90 seconds) and the aerobic or oxidative system (predominant energy source for approximately 2 minutes of exercise).
The organism obtains energy from the utilization of energy substrates (mainly carbohydrates and fats), with or without the participation of oxygen (aerobic and anaerobic metabolic pathways, respectively). The aerobic pathway results in the highest yield for the organism (greater ATP production per unit of substrate) and end products that do not produce fatigue, and is the most important metabolic pathway in exercises of long duration. Its limitation can be found at any point of the system that transports oxygen from the atmosphere until it is utilized in the peripheral mitochondria. It must be taken into account that these 3 energy systems overlap. Thus, the energy pathway predominantly utilized in a physical activity depends on its intensity and duration. Factors that determine the utilization of the energy substrates are the intensity of the exercise (the higher the intensity, the greater the contribution of carbohydrates to energy production), its duration (the longer the duration, the greater contribution of fats), the amounts of carbohydrates prior to exercise and the physical fitness of the individual. Figure 1 shows how, from a certain exercise intensity on, lactic anaerobic metabolism plays a greater role in energy production, despite the fact that aerobic metabolism continues to contribute to the energy supply. It should be pointed out that, when vigorous exercise that surpasses the anaerobic threshold is performed, the maximum concentration of lactate in blood is reached once exercise is discontinued as clearance of the high lactic acid concentration accumulated in muscle takes time.
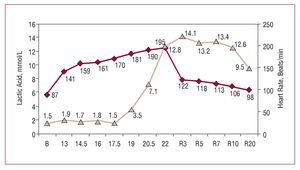
Figure 1. Blood lactate concentration in an elite Spanish male middle distance runner during a field test, performed on the track, that involved an incremental protocol, with an initial velocity of 13 km/hour, increments of 1.5 km/hour every 4 minutes and a final velocity of 22 km/hour. As the subject was a highly trained athlete, his blood lactate level was similar to resting values up to 17.5 km/hour. From this point on, the blood lactate concentration increased, reaching a level of 12.8 mmol/L at maximal effort, and the maximal value (14.1 mmol/L) was recorded at minute 3 of the recovery period. Courtesy of Dr Manuel Rabadán (Centro de Medicina del Deporte, Consejo Superior de Deporte, Madrid, Spain).
The intensity of exercise can be expressed in terms relative to the functional capacity of each individual, as a percentage of the maximum oxygen consumption (VO2max) or in metabolic equivalents (MET). The VO2max indicates the physical work capacity of an individual and reflects, in a general way, the system that transports oxygen from the atmosphere until it is utilized in muscle. The exercise intensity at which the respiratory efficiency is greatest, and at which the energy supply is provided predominantly by aerobic metabolism, corresponds to the aerobic threshold. The effort intensity beyond which the anaerobic metabolism increases substantially and is not compensated for or assimilated by the organism and, thus, fatigue develops, corresponds to the anaerobic threshold. In trained individuals, the physical exertion required for fatigue to develop is greater, indicating that these individuals are capable of performing more vigorous exercise without tiring.4 Figure 2 shows that vigorous exercise of approximately 1 minute requires the participation of lactic anaerobic metabolism. It can be observed that, when vigorous exercise is repeated without allowing sufficient recovery time, lactate builds up in the bloodstream due to a lack of time for the clearance of the high lactic acid concentration accumulated in muscle. Figure 3 shows that, with physical exercise under conditions of metabolic stability, at or under the anaerobic threshold, the blood lactate levels remain constant, even following intense anaerobic exertion.
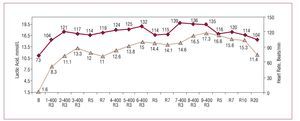
Figure 2. Blood lactate concentration in an elite Spanish male 400-meter dash runner during a field test. The runner performed 3 blocks divided into sets of 400 meters, with 10-minute recoveries between blocks and 4-minute recoveries between sets. The lactate values were measured at minute 3 of the recoveries between sets. Lactate was also determined at minutes 5 and 7 between blocks. This graph demonstrates that high-intensity exercise of about 1 minute's duration demands the participation of anaerobic lactic metabolism. Courtesy of Dr Manuel Rabadán (Centro de Medicina del Deporte, Consejo Superior de Deporte, Madrid, Spain).
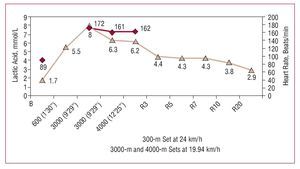
Figure 3. Blood lactate concentration in an elite Spanish male long-distance runner during a field test to confirm the anaerobic threshold. Blood lactate determinations were performed 30 seconds after the 600-meter set and the two 3000-meter sets, as well as 30 seconds after the 4000-meter set and 3, 5, 7, 10, and 20 minutes into recovery. It can be observed that, in contrast to Figures 1 and 2, that at this exercise intensity, below the anaerobic threshold, blood lactate levels decrease after exercise cessation. Courtesy of Dr Manuel Rabadán (Centro de Medicina del Deporte, Consejo Superior de Deporte, Madrid, Spain).
Depending on the type of muscle contraction, physical exercise is classified as dynamic, or isotonic, or static, or isometric. Dynamic exercise is that in which there is successive contraction and relaxation of the muscle fibers, for example, in running or swimming. Given the characteristics of these activities, they are usually referred to as "aerobic." Static or isometric exercise is understood to be that in which stress is generated in the muscle fibers, without important changes in muscle length (the most characteristic example is maintaining the position during weight lifting). In general, it involves vigorous exercise, and is usually not very prolonged. As oxygen consumption is low, this type of exercise is predominantly "anaerobic." In reality, in most types of exercise, the muscle activity is mixed and their classification is based on the predominant type of contraction.
Physical activities can be performed at different degrees of intensity and, thus, the energy expenditure changes according to the intensity and duration of the activity. Moderate physical fitness enables the individual to perform activities that require an energy expenditure of around 150 kcal/day, which corresponds to activities of an intensity of between 3 and 6 MET. These include, for example, brisk walking (4.8 to 6.4 km/hour), cycling at 13 km/hour, moderate-effort swimming, golf, lawn mowing, or housecleaning.5 Table 1 provides examples of physical activities of daily living that result in an expenditure of around 150 kcal/day or 1000 kcal/week for an adult male weighing approximately 70 kg. Since these activities can be performed at different intensities, there is an inverse relationship between the duration and the intensity of the exertion. Table 2 shows the time required to achieve an energy expenditure of 150 kcal in different sports, according to the body weight of the individual, the type of activity, and its intensity.
BENEFITS OF EXERCISE
Aerobic physical activity, that is, of long duration, mild to moderate intensity (individualized) and performed regularly, produces a series of adaptations of differing nature (muscular, osseous, metabolic, respiratory, and cardiovascular) that produce the health benefits. The training associated with sports predominantly involving dynamic exercise and endurance induces morphological and functional cardiovascular adaptations: reduced heart rate and increases in chamber volume, wall thickness, systolic volume, myocardial capillary density (number of capillaries per myofibril), and the dilatory capacity.6 Studies carried out in athletes from different specialties support the concept of a single type of hypertrophy, and have revealed that those involved in sports requiring endurance exhibited a greater increase in left ventricular mass than those in sports requiring strength.7,8
A number of studies performed both in animals and in humans have reported adaptations of the coronary arteries related to the physiological hypertrophy. Structural and metabolic adaptations, increased capillary density proportional to the myocardial wall thickening, increase in the lumen of the coronary vessels, especially in their vasodilatory capacity, and increase in capillary permeability have been observed.6,9,10 All these changes play a role in maintaining adequate myocardial perfusion during physical exercise in order to facilitate blood flow to the cardiac muscle. In patients with coronary artery disease, physical training improves endothelial function of the epicardial coronary vessels and resistance vessels.11 Repeated short sessions of vigorous exercise improve endothelium-dependent vasodilation within 4 weeks, while, on the other hand, regular aerobic exercise prevents age-related loss of vasodilation and normalizes it in previously sedentary middle-aged or elderly men.12 Exercise can favor the production of cytokines that protect against atherosclerosis.13 In patients with ischemic heart disease, training improves cardiac autonomic function, which is reflected in an increased baroreceptor sensitivity and heart rate variability.14
Immune function is also modulated by exercise, with it has a J-shaped relationship. Moderate exercise improves the normal function of cytolytic cells, circulating T and B lymphocytes and the monocytes and macrophages, and, thus, can reduce the incidence of infections and certain types of cancer. In contrast, a session of high-intensity exercise results in a marked decrease in cellular immune function, especially in individuals with a low level of physical fitness.15-17
With respect to mortality, most of the data are provided by observational studies.18 In one of the few available randomized studies, which reports the effects of 2 years of walking in 229 postmenopausal women, after 10 years of follow-up, there was no evidence of a reduction in the mortality rate in the intervention group.19 Other observational studies, however, indicate that among individuals who engage regularly in sports, all-cause mortality is reduced.20-24 In a retrospective study involving a 12-year follow-up, carried out in 10 269 Harvard students, in the men who participated in sports of moderate intensity, the risk of death was 23% lower than in those who were less active. The improvement in survival associated with exercise was equivalent and additive to the other measures taken to improve lifestyle, such as giving up smoking and controlling arterial blood pressure and weight.25 Although moderate levels of physical activity have been shown to be beneficial to health, higher levels appear to provide increased protection.26 Progressive increases in activity have been shown to reduce total mortality. Men and women over 50 years of age who perform intense physical exercise (continuous running or other aerobic activities) present less all-cause mortality than control individuals performing less vigorous exercise. Data from the Framingham study show that moderate and high-level physical activity increase the life expectancy of men by 1.3 and 3.7 years, respectively, as compared with low-intensity activity, and similar results are observed in women, in whom life is prolonged by 1.5 and 3.7 years, respectively.27 However, another study carried out in a population of 302 active volunteers (aged, 70 to 82 years) supports the idea that the daily energy expenditure, measured by means of a metabolic study with radiolabeled water, correlates better with the benefit in terms of mortality than the level of intensity of the physical activity performed.28
With respect to its effects on the cardiovascular system, different studies have demonstrated an inverse relationship between regular exercise and the risk of coronary artery disease, cardiac events, and death.25,29-35 Exercise has beneficial effects on the lipid profile (reduction of low-density lipoproteins and triglycerides, increase in high-density lipoproteins) and on body composition, the aerobic capacity and hemostasis, thus reducing the risk of thrombosis. Exercise improves glycemic control and insulin sensitivity, and prevents the development of type 2 diabetes mellitus in high-risk patients.36
Exercise offers a number of benefits, and a number of studies have demonstrated that it reduces or prevents obesity,37-40 delays weight gain after smoking cessation,41 reduces the risk of gallstone disease,42 and even has a modest protective effect against cancer.43-47 In elderly individuals, it improves their functional status and their autonomy, prevents or delays cognitive deterioration and decreases the incidence of Alzheimer's disease.48-51 The effects of exercise are not only related to the cardiovascular benefits and are summarized in Table 3.
RISKS OF EXERCISE
In addition to bone and muscle injuries, physical exercise has other adverse effects, some involving cardiovascular conditions, such as arrhythmias, sudden cardiac death, or myocardial infarction, and others associated with muscle conditions, such as rhabdomyolysis.52 There is a transient increase in the risk of sudden cardiac death during the performance of vigorous exercise, even in healthy men; however, the absolute risk during a given exercise session is very low, 1/1 510 000 exercise sessions, according to the Physicians' Health Study.53 In this study, over a 12-year follow-up period, there were 122 sudden deaths among 21 481 male physicians who were initially free of cardiovascular disease. The relative risk (RR) of sudden cardiac death during intense exercise or within the subsequent 30 minutes was 16.9 (95% confidence interval [CI], 10.5-27; P<.001); however, regular vigorous exercise attenuated this risk. The same is observed in women, as demonstrated in the Nurses' Health Study,54 in which, during the follow-up of 69 693 women between 1986 and 2004, there were 288 sudden deaths during vigorous exercise, resulting in a RR of 2.38 (95% CI, 1.23-4.6; P=.01). In this population, the total risk was extremely low, with 1 death for every 36.5 million hours of exercise, and, as in men, regular moderate or vigorous exercise had a protective effect (RR=0.41 in women who exercised for 4 hours a week or more, as compared to sedentary women).
The mechanisms postulated for sudden death during exercise include arrhythmias, especially tachycardia or ventricular fibrillation, and acute coronary ischemia secondary to plaque rupture, and coronary thrombosis. Coronary spasm has also been reported to be a causal mechanism in diseased coronary arteries. However, regular moderate or vigorous exercise has an attenuating effect on the risk of atrial and ventricular arrhythmias during a session of vigorous exercise, in part due to the improved oxygen delivery to the myocardium and the reduction in sympathetic tone.
On the other hand, during strenuous physical activity, there is a transient increase in the risk of acute myocardial infarction (AMI), especially among individuals who do not exercise regularly,52,55,56 In a study of 1194 men diagnosed as having AMI, vigorous exercise (activity 36 MET) was involved in 7.1% of the cases at the onset of the infarction. The RR during vigorous exercise or within the following hour was 2.1, and was higher in patients who exercised less than four hours weekly on a regular basis than in those who exercised more than 4 hours a week (RR=6.9 and RR=1.3, respectively; P<.01).55 In another report, the RR during exercise was 10-fold higher than the risk under other circumstances.56
Finally, although vigorous exercise has been associated with a number of harmful effects (episodes of bronchoconstriction, hyperthermia or hypothermia, dehydration, urticaria, and even anaphylaxis), the beneficial effects of regular exercise on health far surpass the risks.
THE LACK OF EXERCISE AS A HEALTH PROBLEM
Inadequate physical activity is considered to be an independent risk factor for coronary artery disease. Approximately 12% of the total mortality in the United States is related to the lack of regular physical activity, and inactivity is associated with at least a 2-fold increase in the risk of a coronary event, with a RR similar to that of hypertension, hypercholesterolemia, or smoking.57 It is estimated that there are around 200 000 deaths/year due to ischemic heart disease, cancer, or type 2 diabetes mellitus related to a sedentary lifestyle.20,58-60
In contrast, regular physical activity and cardiovascular fitness reduce the total mortality. In the prospective study carried out by Blair et al29 to assess the relationship between changes in physical fitness and risk of mortality, the authors performed follow-up studies in 9777 men for a mean period of 5.1 years. All of the subjects underwent 2 medical examinations, at a mean interval of 4.9 years, which included maximal exercise tests, to determine the changes, or deterioration in physical fitness. Throughout follow-up, there were 223 deaths corresponding to all-cause mortality and 87 cardiovascular deaths. The highest age-adjusted, all-cause mortality rate was observed in men who were unfit at the time of both examinations (122/10 000 man-years). In contrast, the lowest mortality rate was found in the men who were physically fit at both examinations (39.6/10 000 man-years). Among the individuals who had improved their level of fitness during the interval between examinations, the age-adjusted death rate was 67.7/10 000 man-years. This corresponds to a reduction in mortality risk of 44% (95% CI, 25-59) with respect to those who were found to be unfit at both examinations. Improvement in physical fitness was associated with a lower age-adjusted mortality rate, with health status and with other risk factors of early mortality. For each increase by 1 minute in treadmill time between the 2 exercise tests, there was a decrease of 7.9% in the risk of mortality (P=.001). Thus, this test shows that men who maintain or improve their good physical condition have a lower likelihood of dying from all causes and from cardiovascular disease than those who remain in poor physical condition.
Despite the fact that the world is becoming aware of this relationship and that increasing numbers of people are undertaking exercise programs, in a superdeveloped country like the United States, 25% of the adults still do not engage in leisure-time physical activity and only 15% follow the recommendations of 30 minutes of moderate exercise 5 days a week.61 Population surveys continue to indicate that the levels of physical activity are low in the United States. A recent study has reported the prevalence of poor physical fitness in the population ranging in age between 12 and 49 years and its relationship to the risk factors of ischemic heart disease. A cohort from the National Health and Nutrition Examination Survey 1999-2002, including 3110 adolescents between the ages of 12 and 19 years and 2205 adults from 20 to 40 years of age, all free of cardiovascular disease, who underwent treadmill exercise testing to achieve at least 75% to 90% of their age-predicted maximum heart rate. Low levels of fitness were detected in 33.6% of the adolescents (approximately 7.5 million United States adolescents) and in 13.9% of the adults (approximately 8.5 million United States adults). The prevalence was similar among girls and boys (34.4% and 32.9%, respectively; P=.40), but was higher in adult women than in men (16.2% and 11.8%, respectively; P=.03). The body mass index (BMI) and waist circumference were inversely related to fitness in all the age and sex groups. The total cholesterol and systolic blood pressure were higher and high-density lipoprotein cholesterol(HDL-C) concentrations were lower in the group with low levels of fitness, as compared to the high fitness group.62
On the other hand, the increase in childhood obesity in industrialized countries is attributed to the lack of physical activity that is becoming increasingly evident among children. It should be noted that the sedentary lifestyle can begin in early childhood, as reflected in the study carried out by Reilly et al63 in Scottish 3-year-olds, with a follow-up study at 5 years. The behavior of these children was sedentary 79% of the time at age 3 and 76% of the time at age 5, and the median time occupied in physical activity was only 2% of the time monitored at age 3 years and 4% at the age of 5. In Spain, the authors of different studies affirm the importance of a sedentary lifestyle with respect to childhood and adolescent obesity, and cardiovascular risk.64,65
EXERCISE IN PRIMARY AND SECONDARY PREVENTION
The evidence provided in clinical studies to establish the benefits of exercise in primary and secondary prevention is inadequate due to a number of factors. The adherence in randomized studies is not good because, on the one hand, from an ethical or practical point of view, it is difficult to assign active individuals who exercise regularly to the control group and, on the other, after several years of follow-up, the level of exercise could come to be similar in the 2 groups. Moreover, these studies usually include few patients and, therefore, the significance of the benefit they show is limited. Thus, not all the reports reach the same conclusions concerning the effect of exercise on the reduction of cardiovascular risk. Oja,66 in his review of 19 observational studies and 15 randomized studies involving primary prevention in an inactive healthy population that included middle-aged and elderly men and women, did not find a clear correlation. On the one hand, the results of cross-sectional and longitudinal studies indicate a gradual dose-response relationship between the amount of physical activity and all-cause mortality, stroke, and different coronary risk factors, with similar effects in men and women. On the other hand, randomized studies have demonstrated a clear and gradual response to the amount of exercise of the oxygen consumption, but not of the risk factors. In consequence, most of the conclusions concerning the benefits of exercise are drawn from observational studies in which the patients who exercised regularly presented a significantly lower incidence of ischemic heart disease and a reduced risk of primary cardiac arrest.67
A number of studies demonstrate an inverse relationship between physical activity and energy expenditure, physical exercise, and fitness, and the risk of ischemic heart disease and death, both in men and women of different ethnic groups and from different countries.22,25,26,29,30,33,68-75 The benefit of exercise in relation to the risk of myocardial infarction was demonstrated in the INTERHEART study68 with patients from 52 countries. Regular physical activity was associated with an odds ration (OR) for a first myocardial infarction of 0.86, with a population attributable risk of 12%. This beneficial effect was observed in men and women, young and old, and in every country studied. However, there is no consensus as to the amount and intensity of the physical activity required in primary prevention. The incorporation of physical activities of moderate intensity into the lifestyle appears to provide benefits comparable to those associated with a structured exercise program. A number of studies have proposed to verify the influence of exercise on cardiovascular health in different groups of patients. Among these studies, that of Sesso et al,69 carried out in the population involved in the Harvard Alumni Health Study, is of particular interest. The study consisted of the 16-year follow-up of 12 516 men with a mean age of 57.7 years for the purpose of determining the effect of physical activity on cardiovascular risk. Weekly physical activity was measured on the basis of the energy expended per week in kilojoules or kilocalories (4.2 kJ are equal to 1 kcal) from stairs climbed, blocks walked and recreational or sports activities over the preceding year. The mean physical activity level was 8362 (8215) kJ/week, and 74.1% of the participants engaged in recreational or sports activities. Most of the energy was expended in moderate (4 to 6 MET) or intense (36 MET) activities, for a contribution of 37.4% and 56.1%, respectively, to the total energy expended per week. During follow-up, there were 2135 cases of coronary heart disease, 576 of which corresponded to myocardial infarction, 512 to angina pectoris, 207 to coronary revascularization, and 840 to cardiac death. In the age-adjusted model, there was an L-shaped association between the levels of physical activity and the risk of coronary heart disease, with a reduction in risk of 23% for a physical activity resulting in an energy expenditure of 4200 kJ/week, and there was no additional reduction in risk for expenditures over 8400 kJ/week. Moreover, it was observed that the association with cardiovascular risk factors did not modify the inverse relationship between physical activity and cardiovascular risk. Men aged less than 60 years with an energy expenditure greater than or equal to 4200 kJ/week had lower levels of coronary risk than those who were inactive. This energy expenditure is achieved by performing, on a daily basis, activities such as brisk walking, recreational swimming or cycling, or doing home repairs, or yard work for 30 minutes a day. This study also indicates that vigorous activities are associated with cardiovascular disease risk reduction, whereas, with moderate or light activities, there is no clear reduction of the risk. Moreover, a physically active lifestyle can reduce the probability of the development of concomitant risk factors. In particular, men aged 60 years or more whose energy expenditure is greater than or equal to 4200 kJ/week exhibit only small increases in cardiovascular risk in the presence of coronary risk factors. According to the results of this study, the total weekly physical activity (>4200 kJ/week) is associated with the most marked reduction in cardiovascular risk. Moderate or light activities, which can be measured with less precision, result in a nonsignificant reduction in cardiovascular risk of 10%.
Thus, in other reports, like that of Lee et al,72 the level of intensity of the exertion as perceived by the individual is employed as a criterion for its assessment. This also appears to affect risk in that those who feel that they are performing moderate or intense exercise have a lower cardiovascular risk than those who perceive their activity to be light or less intense.
In the assessment of the population of women, we have to refer to the work of Stampfer et al,76 who followed 84 129 women who were free of cardiovascular disease, cancer, and diabetes for 14 years. There were 1128 coronary events (296 deaths and 832 nonfatal AMI). Low-risk women (nonsmokers with a BMI under 25, who engaged in moderate or vigorous exercise for over 30 minutes a day, consumed less than 10 grams of alcohol a day and a diet that promoted cardiovascular health) had a RR of coronary heart disease of 0.17. Taking these data into account, if the entire population had been low-risk, 82% of the coronary events could have been avoided.
In another study involving 70 000 postmenopausal women, both walking and vigorous exercise were associated with a reduction in the risk for cardiovascular events, irrespective of age, race, or BMI.73
However, physical fitness and cardiorespiratory capacity have a strong and gradual inverse association with total mortality, both cardiovascular and noncardiovascular. The VO2max and the duration of exercise during the exercise stress test are strong predictors of mortality. In the follow-up of 1294 Finnish women with no cardiovascular or pulmonary disease or cancer over a 10-year period, the RR of death was found to be related to the VO2max. After adjustment for age, the years of follow-up, the absence of smoking and alcohol consumption, the RR in the unfit group (VO2max <27.6 mL/kg/min) was 2.76 for all-cause mortality and 3.09 for cardiovascular deaths, with respect to men who are physically fit (VO2max >37.1 mL/kg/min). With respect to the duration of the exercise stress test, in the group in which it was less than eight minutes, the RR were 3.94 and 4.54, respectively, as compared to those who had withstood over 11 minutes.70 The lowest cardiorespiratory fitness is associated with a risk comparable in importance to those reported for high systolic blood pressure, smoking, obesity, and diabetes.
The intensity of exercise necessary to obtain cardiovascular benefits was evaluated in a cohort of 44 452 men ranging in age between 40 and 75 years, enrolled in the Health Professionals' Follow-up Study.74 During follow-up of 475 755 patient-years, there were 1700 new cases of ischemic heart disease. The total physical activity, running, weight training, and rowing correlated with a significant decrease in cardiovascular risk. Men who ran for at least an hour a week had a 42% reduction in risk. Those who trained with weights for at least 30 minutes a week and those who rowed for at least 1 hour a week achieved a risk reduction of 23% and 18%, respectively. The RR corresponding to moderate intensity (4 to 6 MET) and to high intensity (6 to 12 MET) exercise were 0.94 and 0.83, respectively, with respect to that of low intensity (<4 MET). Walking briskly for 30 minutes a day was associated with a risk reduction of 18%. The duration of the exercise session required to reduce cardiovascular risk is not clear; although the length of time does not appear to have any influence, the amount of energy expended in each session does play a role to the point that the expenditure of a large amount of energy reduces age-adjusted risk.71 After adjusting for age, the functional capacity of an individual, measured in MET, during a maximal exercise test is a strong predictor of mortality in men with and without cardiovascular disease. For each MET by which the exercise capacity is increased, survival is increased by 12%.75 Similar data were reported in the analysis of 3043 individuals (1431 men and 1612 women) from the Framingham study, who underwent treadmill exercise testing to determine its usefulness in predicting coronary heart disease. The patients were followed for 18.2 years and the variables employed to evaluate risk were ST segment depression, the inability to reach 85% of the predicted maximum heart rate and exercise capacity. The latter variable was associated with the lowest risk of heart disease, in such a way that, for each increment in MET, as an expression of exercise capacity, the risk was reduced by 13%.77 Similar results were obtained in other studies involving asymptomatic women, in which the follow-up of cardiovascular complications revealed an inverse relationship with respect to fitness, expressed as the exercise time during the exercise stress test.78,79
With respect to the effects of exercise on cardiovascular risk factors, poor fitness appears to be associated with the development of diabetes, hypertension, and metabolic syndrome both in young and middle-aged subjects. The CARDIA study80 involved the follow-up of 5000 men and women between the ages of 18 and 30 years over a 15-year period. During follow-up, the incidences of new cases of diabetes, hypertension, and metabolic syndrome were 0.3%, 1.3%, and 1% per year, respectively. Physically unfit individuals (below the 20th percentile) had a 3 to 6-fold higher probability of developing these risk factors than those who were physically fit (above the 60th percentile). In another study, carried out in a population of 9007 men (age, 44 [9] years; BMI, 25 [3]) and 1491 women (age, 44 [9] years; BMI, 22 [2]), the authors carried out a prospective analysis of fitness and the metabolic syndrome. During follow-up (mean, 5.7 years), 15% of the men and 3.8% of the women developed the metabolic syndrome, there being a significantly lower probability in the middle and upper thirds in terms of fitness in men (hazard ratios, 0.74 and 0.47, respectively) and in the upper third in women (hazard ratio, 0.37).81
Finally, with respect to secondary prevention, different studies point out that exercise benefits patients with ichemic heart disease.72,82-85 Light or moderate recreational physical activity of at least 4 hours a week or walking over 40 minutes a day reduces the incidence of both all-cause death and cardiovascular death in patients with coronary heart disease.82 In a meta-analysis carried out in 2005 by Clark et al,83 63 randomized studies were reviewed for the purpose of evaluating the efficacy of different cardiac rehabilitation programs, with and without exercise, in patients with documented ischemic heart disease (postinfarction in the majority). These authors observed that exercise alone produced a significant reduction in all-cause mortality (6.2% vs 9%; RR, 0.72; 95% CI, 0.51-0.95).
The reported observations have led the majority of the medical and cardiology societies to promote exercise as part of the change toward a lifestyle that improves cardiovascular health.86,87 Thus, they recognize that adequate physical activity is valuable as a complementary therapeutic measure for the control and treatment of coronary heart disease and cardiovascular risk factors.
DIFFERENT RECOMMENDATIONS AND GUIDELINES FOR PHYSICAL ACTIVITY
Exercise in Hypertensive Individuals
In hypertensive patients, the VO2max reached during an exercise stress test is of prognostic significance. Low VO2max levels are significantly and independently associated with a higher incidence of cardiovascular events and total mortality in hypertensive patients. Thus, the beneficial effect of exercise goes beyond the mere decrease in arterial blood pressure.
Exercise programs stressing the dynamic component prevent the development of hypertension or lower the blood pressure in normotensive and hypertensive adults. However, the effect of physical activity on arterial blood pressure is more marked in hypertensive patients, with a mean reduction of the systolic and diastolic arterial pressure of 6 to 7 mm Hg, versus 3 mm Hg in normotensive individuals.88 There is no evidence that age, weight, or race influence of blood pressure-lowering effect of training, and, although the influence of sex is unclear, the pressure-lowering response appears to be slightly less marked in women.
With respect to the features of the training program, it seems that all types of exercise, including weight training cycles, reduce arterial blood pressure in hypertensive individuals. To date, there does not appear to be consensus as to the most adequate exercise intensity, although moderate-intensity exercise results in similar or even more marked decreases than those produced by high-intensity exercise. With regard to the type of exercise, most authors agree on the efficacy of programs that include aerobic activities such as walking, jogging or running, swimming, cycling, or dancing at a moderate intensity (40% to 60% of the VO2max or 60% to 75% of the maximum heart rate), with sessions of 30 to 45 minutes at least 4 or 5 days a week.88,89 Circuit weight training can be performed, with a set of eight to ten repetitions per exercise, with an intensity of 40% to 50% of one repetition maximum (1 RM), in such a way as to not surpass systolic arterial pressure and diastolic arterial pressure values of 150 mm Hg and 100 mm Hg, respectively. Aside from ensuring the desired antihypertensive effect, combined training programs that include both resistance and strength exercise make training more enjoyable and reduce dropouts. Table 4 shows the characteristics of an exercise program for patients with hypertension.
Exercise and Hypercholesterolemia
The lipid response to aerobic exercise in untrained men appears to be independent of baseline cholesterol levels and may, in part, be due to the increase in lipoprotein lipase activity. Immediately after a session of aerobic exercise at an intensity of 70% of VO2max, there is a decrease in total and low-density lipoprotein cholesterol (LDL-C), which return to baseline concentrations 24 hours later.90 The reduction in the serum triglyceride concentration and the increase in the HDL-C and HDL3-C fractions, as well as the increase in lipoprotein lipase activity, persist over a longer period of time (at least 48 hours). There are very few studies that demonstrate the response of lipid levels during a training program, and the effects can vary in relation to the nature of the dyslipidemia. High cholesterol and triglyceride levels and the total cholesterol/HDL-C ratio are correlated with greater decreases following exercise, whereas low HDL-C concentrations are associated with less marked increases in the response to exercise.
With respect to age, to obtain improvements in lipemia, elderly individuals require more prolonged exercise programs than young people. In terms of sex, there are no sex-related differences in the response of triglycerides to exercise, but the response of HDL-C is more attenuated in women. Athletes have been shown to have higher HDL-C and lower LDL-C concentrations than those observed in individuals with a sedentary lifestype91; however, the intensity with which the exercise program should be carried out in order to obtain benefits in terms of the lipid profile is a parameter that has yet to be established. It has been observed that, after a 12-week period of aerobic training at moderate to high intensity, there are positive changes in the lipid profile, although it has not been possible to determine the point at which this training begins to be beneficial.92
On the other hand, the frequency and duration of the exercise session and the time elapsed between the initiation of the training program and the detection of benefits in terms of the lipid profile appear to be more important. In general, sessions of lower intensity and longer duration are recommended to ensure a great enough caloric expenditure. In young populations, periods of 6 to 12 months are sufficient to achieve increases in HDL-C, but in adults aged 50 years and over, they should be longer, at least 2 years, in order to produce adaptations of lipid metabolism, although these individuals experience an improvement in fitness and slight changes in their HDL-C levels from the start of the regular exercise program. Table 5 shows the characteristics of a training program to improve the lipid profile.
Exercise and Type 2 Diabetes Mellitus
With age, there is an increase in the prevalence of type 2 diabetes mellitus associated with the loss of muscle mass, and it is estimated that at least 25% of its incidence is attributable to a sedentary lifestyle. Different studies have demonstrated a decrease in the incidence of type 2 diabetes mellitus among physically active individuals, and the Finnish study on the prevention of diabetes has estimated a risk reduction of 58% when active individuals are compared with those who are sedentary.93 The effects of aerobic exercise on glycemic control are inconsistent, and it seems that only certain subgroups benefit, such as those patients with type 2 diabetes managed with dietary therapy who achieve good glycemic control. In patients with type 2 diabetes mellitus, beneficial effects of aerobic exercise (75% of the VO2max) performed for 45 minutes twice a week, plus 25 minutes of 2-minute sets (85% of the VO2max) with 3-minute recovery periods (50% of the VO2max) once a week, are observed after 12 months. The benefits consist of a loss of nearly 50% of abdominal fat and an increase in muscle mass of 23%, with a significant decrease in the glycosylated hemoglobin (HbA1c) levels and an increase in insulin sensitivity. Moreover, strength training produces an increase in muscle strength, with a notable improvement in HbA1c concentrations, and there is a close relationship between glycemic control and muscle mass. These findings are in accordance with the pathogenesis of this type of diabetes and, thus, strength training should be included in exercise programs aimed at glycemic control. A 12-month controlled exercise program reduces BMI and decreases the HbA1c level by 0.26%, systolic arterial pressure by 7.7 mm Hg, total cholesterol by 0.33 mmol/L, and fibrinogen by 0.28 mmol/L, whereas the simple recommendation to exercise is not accompanied by changes in physiological or biochemical variables.93 Consequently, for a training program for type 2 diabetic patients to be effective, it should include moderate-intensity dynamic exercise and high-level strength training in order to improve cardiorespiratory capacity, muscle strength and different physiological and biochemical parameters.
Table 6 shows the features of an optimal exercise program for type 2 diabetic patients.
Exercise and Obesity
According to the Framingham study, obesity is a minor coronary risk factor, and does not appear to increase the risk of mortality in men who are physically fit. The cardiovascular benefits obtained by increasing physical activity are greater than those of dietary control to lose weight. Physical training in association with a hypocaloric diet reduces body weight, preferentially the percentage corresponding to fat, as it increases resting energy expenditure and metabolic indices. Weight loss is associated with an improvement in insulin resistance, reduction of inflammatory markers like C-reactive protein, decreases in diastolic and systolic arterial pressures, and improvement in the lipid profile.94
Moreover, physical activity can also counteract the increase in fat mass that occurs with age. In fact, older women athletes have a significantly lower amount of body fat than sedentary women of the same age (15% vs 27%). However, it should be taken into account that, to lose a significant amount of body fat, it is necessary to maintain a training period of at least 20 minutes a day, 3 days a week, with an intensity and duration sufficient to burn 300 kcal per session. Aerobic activities (walking, running, cycling) are those most widely recommended, and all of them are equally effective in reducing body fat, whereas anaerobic activities (weight lifting) increase muscle mass, but have a less marked effect on the amount of fat.
CONCLUSIONS
The balance between the risks and benefits of engaging in physical activity clearly favors the benefits, especially when it is performed regularly, although there appears to be a minimum weekly energy expenditure threshold required to reduce cardiovascular risk. Physical activities of moderate to high intensity with an energy expenditure of 1000 kcal per week or more result in the greatest benefit. In contrast, a sedentary lifestyle is associated with a 30% higher risk of ichemic heart disease than that reported for dyslipidemia or hypertension, surpassed only by smoking. Consequently, exercise should be considered the cornerstone on which to base changes in lifestyle aimed at the prevention of cardiovascular disease.
Section Sponsored by Laboratorio Dr Esteve
Correspondence:
Dra. A. Boraita.
El Greco, s/n. 28040 Madrid. España.
E-mail: araceli.boraita@csd.mec.es