Keywords
INTRODUCTION
Chest pain is one the most frequent causes for attending clinics in Spain.1,2 Results of initial studies conducted in different countries showed that a high percentage of high-risk patients were incorrectly diagnosed; notably, 5%-10% of patients with an acute myocardial infarction (AMI) were discharged from emergency departments.2-9 In recent years we have witnessed the setting up of chest pain units which use algorithms to generate management protocols for patients attending emergency departments with suspected acute coronary syndrome (ACS).10-13
Numerous risk evaluation scales (scores), based on clinical characteristics and laboratory markers, have been proposed for this type of patient.14-18 One of the most widely used is the TIMI Risk Score (TRS).19-22
Despite the proven usefulness of the TRS in high-risk non-ST segment elevation ACS, its value in the general context of non-selected patients with chest pain is not fully defined.
Thus, the objective of the present study is to test the efficacy of the TRS as an index of risk stratification in a non-selected population attending emergency departments for chest pain.
PATIENTS AND METHOD
From May thru October 2001, 1334 (3.2%) of a total of 41 409 patients attending our emergency department had non-traumatic chest pain. We excluded 80 patients with ST-segment elevation ACS, which left a final study population of 1254 consecutive patients.
Initially, patients were evaluated by the emergency department physician and the opinion of a cardiologist was requested if a cardiac cause was suspected. Patients with non-cardiac chest pain were discharged or treated according to the diagnosis which was reached. After patient evaluation, the cardiologist decided to admit or discharge on the basis of clinical, electrocardiographic and laboratory data (creatinkinase [CK], MB isoenzyme of creatinkinase [CK-MB], and troponins) over the first 24 hours.
For each patient, characteristics of the pain, relevant personal antecedents, cardiovascular risk factors, previous treatment, and TRS based on 7 clinical variables were recorded.19 We prospectively calculated TRS for each patient, with scores in the 0 to 7 point range based on the simple arithmetic sum of each of the following characteristics: age ≥65 years; presence of 3 or more traditional risk factors (arterial hypertension, hypercholesterolemia, diabetes mellitus, smoking, and family history of early ischemic heart disease); previous history of significant coronary disease (stenosis ≥50%); administration of aspirin in the last 7 days; at least 2 episodes of angina in the last 24 hours; elevation of cardiac necrosis markers and ≥0.5 mm ST-segment alteration.
We defined 2 groups of patients:
- Group A: patients discharged from the emergency department.
- Group B: patients admitted to the cardiology department.
Patients hospitalized received antiplatelet treatment (aspirin, 100-300 mg/day; clopidogrel, 75 mg/day, with a 300 mg loading dose) and subcutaneous enoxaparin (1 mg/kg every 12 hours) unless contraindicated. Other drugs, such as beta-blockers, calcium antagonists or nitrates were administered in different combinations to control symptoms.
Follow-up
The 6-month follow-up included 1190 patients (94.9%) and we collected data on control of risk factors, compliance to treatment regimen, presence of symptoms, functional class, need for readmission, revascularization, occurrence of events such as AMI or death, and major or minor bleeding. The composite endpoint was defined as occurrence of any of the following: coronary revascularization, AMI, or cardiovascular death.
Statistical Analysis
Statistical analysis was performed with SPSS 11.0 (SPSS Inc. Chicago, Illinois, USA). Data were given as mean ± standard deviation (SD), range or frequency (percentage). We used the Kolmogorov-Smirnov test to evaluate the normality of the distribution of continuous quantitative variables in <30 patients. We used the Student t test to compare continuous quantitative variables in 2 groups when these were normal or there were >30 cases; otherwise we used a nonparametric test (Mann-Whitney U). We used the Pearson χ2 test to compare 2 dichotomous variables if the number of expected observations in all cells was >5, and the Fisher exact test for 2 to 5 expected observations. The logistical regression test was used to evaluate the relationship between significant variables and events. Statistical significance was set at P<.05.
RESULTS
The prevalence of chest pain as a motive for attending the clinic was 3.2%. Baseline patient characteristics, both overall and for each of the 2 groups are summarized in Table 1. Hospitalized patients were significantly older and mostly male. They presented a greater number of risk factors, higher incidence of infarction or previous revascularization and cardiovascular treatment. Typical chest pain suggestive of angina was present in 64% of patients hospitalized versus 2.4% of patients discharged from the emergency department. Pain was considered non-specific in 61% of these patients. Chest pain was accompanied by dynamic electrocardiographic changes in 17% of hospitalized patients and 32% had troponin I elevation. Electrical changes (n=3) or elevated troponin I levels (n=4) were present in less than 0.5% of patients discharged; both abnormalities were attributed to non-ischemic alterations or occurred in patients not considered for admission.
The TRS distribution of the patient population is described in Table 2: 4.9% (n=45) with TRS >3 were discharged and 24.5% (n=84) with TRS=0-1 were hospitalized.
Dual antiplatelet treatment with aspirin and clopidogrel was administered to 180 patients (52.5%), this was associated with tirofiban in 43 (12.5%). We tested 45% of hospitalized patients for ischemia (37% were positive) and 47% underwent coronary angiography; single vessel disease was found in 49 patients (30%); multivessel disease in 78 (48%) and absence of significant lesions in 34 (21%).
Table 3 shows in-hospital events at 6 months for the study population. Among hospitalized patients, only 17 (5%) of those who received in-hospital revascularization underwent surgery. Of the 7 cardiovascular deaths, 3 were sudden, 2 due to heart failure and 2 followed cardiovascular surgery (1 intestinal ischemia and 1 septic shock). Other 2 patients died of non-cardiac cause. Final diagnoses of the 343 hospitalized patients were: myocardial infarction, 49 (14.3%); unstable angina, 129 (37.6%); secondary angina, 21 (6.1%); other cardiac causes, 50 (14.6%); and non-cardiac pain, 94 (27.4%). Patients with a final diagnosis of ACS presented significantly higher TRS than patients whose final diagnosis was not ACS (3.3±1.3 vs 2.0±1.2; P<.001).
We achieved full clinical follow-up in 1190 patients (94.9%). At 6 months, 9.6% of hospitalized patients had presented a composite endpoint versus only 1.3% of those discharged from the emergency department; moreover, all the parameters evaluated were significantly greater. Among the patients discharged from the emergency department, 45 (5.3%) were hospitalized within 6 months; 9 (1.1%) required coronary revascularization; 5 (0.6%) presented AMI; 2 (0.2%) died due to cardiovascular causes (of these, one 76 year-old man with TRS=3 died of heart failure and one 89 year-old man with TRS=5 of sudden death). Patients with high TRS (≥4) presented significantly greater risk of composite endpoint at 6 months (5 [11.1%] vs 7 [0.9%]; P<.001).
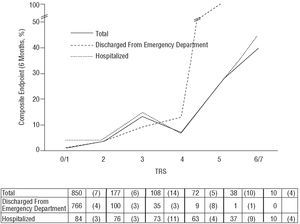
Table 4 shows the relationship between TRS and cardiac events at 6 months in the 2 study groups. Figure shows endpoint percentages according to the TRS for all patients and by group. The TRS was a good predictor of risk gradient. Patients with higher scores more frequently presented composite endpoints at 6 months (from 3.6% to 40.0% for TRS of 0/1 and 6/7, respectively, in hospitalized patients and from 0.5% to 100% for TRS of 0/1 and 5, respectively, in patients discharged from the emergency department). The relative risk of the composite endpoints in the first 6 months of follow-up per unit TRS increase among hospitalized patients was 1.72 (95% confidence interval [CI], 1.32-2.24; P<.001) and 3.63 among patients discharged from the emergency department (95% CI, 2.20-6.00; P<.001).
Thus, we observed a significant relationship between characteristics of the pain leading to attend the clinic and events observed in patients discharged from the emergency department. Of these, 5 (0.7%) with non-specific chest pain, 4 (3.9%) with suggestive pain and 3 (13.5%) diagnosed with angina presented composite endpoints at 6 months (P<.001 for non-specific vs suggestive/angina). However, we did not find this association in the 4 (11.4%) hospitalized patients with non-specific chest pain versus the 30 (9.9%) patients with suggestive/angina pain who presented composite endpoints (P=.8).
DISCUSSION
This study of a non-selected population with chest pain confirmed the TRS as a valuable prognostic predictor and it establishes a risk gradient from 0.5% for patients with low TRS (0/1) up to 40% for patients with high scores (6/7). This risk scale had a high prognostic value both among hospitalized and discharged patients. The present study is the first to have evaluated this in a large, prospective, consecutive, non-selected group of patients attending an emergency department.
Different methods of prognostic stratification based on clinical history and complementary tests for management of ischemic chest pain have been developed. Risk prediction algorithms such as those proposed by Goldman et al7,8 or Pozen et al5 prove complex and are little used. Braunwald's classification of unstable angina, much used in the last decade, has recently been revised to include cardiac enzymes levels.14 In Spain, the PEPA (Proyecto de Estudio del Pronóstico de la Angina; study project on the prognosis of angina) study, defined from clinical and electrocardiographic data the 90-day risk of death and infarction in patients with ACS without ST-segment elevation.18
The Thrombolysis in Myocardial Infarction (TIMI) study risk score has become widely used as a tool to stratify prognosis of patients with ACS with and without ST-segment elevation.19,21,23,24 The TRS provides a simple schematic evaluation with great prognostic capacity, based on 7 variables that can be easily obtained at the patients' bedside. This scale was developed thru the retrospective application of multivariate statistical analysis on the populations of 2 heparin trials: TIMI-11B25 and ESSENCE (Efficacy and Safety of Subcutaneous Enoxaparin in Unstable Angina and Non-Q-wave Myocardial Infarction).26 Later, it was validated in studies such as CURE,27 PRISM-PLUS,28 or TACTICS-TIMI 1829 and has proven valid to predict prognosis and response to new treatments and interventions. However, little is known about its application in daily clinical practice beyond the trials in which it was developed and in non-selected populations, where presence of electrical changes or elevation of cardiac enzymes were frequent inclusion criteria.23,29,30 Bartholomew et al31 evaluated 245 consecutive patients hospitalized for chest pain and found the TRS to be a potent predictor of events. The very high 30-day rate of these events, 20% for TRS=2 and >50% for TRS≥5, reflects a high-risk population, higher even than that of the original TIMI study (>70% of patients with electrical changes and 30% presenting signs of heart failure). This contrasts with the lower percentage of events in the present study, which includes low-risk patients discharged after examination in the emergency department. Sample size and differences in the profiles of patients who attend different centers could partially explain these discrepancies.
The fact that our population is heterogeneous and non-selected could explain why the general prognosis is better even than in Antman et al's original registry,19 even though they also included high-risk patients (mean age, 54 years, 42% with hypertension, 17% with diabetes, 11% with previous infarction, 10% with previous revascularization, and 9% with high troponin levels). The high percentages of antiplatelet treatment (52% with a combination of 2 drugs and 12% with a combination of 3) and revascularization (34%) possibly lowered the rate of events found among hospitalized patients. This could reflect the impact of recent studies, such as CURE27 and TACTICS,29 on daily clinical management. This is reinforced by the similarity of our results with those of the validation of TRS in CURE,32 which compared the efficacy of combined antiplatelet treatment using aspirin and clopidogrel with aspirin alone on prognosis in 12 562 patients with ACS. Results showed 3% of events at 9 months with TIMI=0/1 scores and 19% with TIMI=6/7 in the aspirin and clopidogrel group, although revascularization was not included in the composite endpoint. Our 4.5% (>1.5%) rate for bleeding is similar to that of CURE and other studies such as the TOPSTAR trial33.
Limitations
Although the study population is large (1254 patients), the TRS distribution is not homogeneous. Only 10 patients (0.8%) presented TRS=6/7 and 38 (3.0%) TRS=5. This may have influenced the frequency of events in this group (Figure) even though this distribution is the most common in non-selected populations. It is important to note differences in the determination of enzymes in our study by comparison with the original and later validation trials. We performed serial evaluations of troponin concentrations in all patients as well as analyzing CK and CK-MB. Greater sensitivity of troponin concentrations34,35 could have conditioned TRS differences. Diagnosis of infarction using new criteria including elevated troponin concentrations could cause differences in the calculation of events. As in the original study, quantitative data on troponin concentrations was not included, nor was the contribution of other biological markers, such as C reactive protein, evaluated. Finally, it may not be possible to extrapolate results of the present single-center study to contexts with differences in population and clinical management.
Figure. Composite endpoint (revascularization/infarction/death) at 6 months according to the TIMI Risk Score (TRS) for hospitalized patients, patients discharged from an emergency department and total patients. Values represent number of patients with number of events in parentheses. Relative risk total for each increase in TRS=2.32 (95% confidence interval [CI], 1.91-2.82; P<.001).
CONCLUSION
The TIMI risk score is an efficient tool in the stratification of risk of cardiovascular events in patients attending an emergency department for chest pain. The TRS enables us to identify those high-risk patients who would benefit from admission and active treatment, individualized to suit each patient's needs. Patients with TRS=0/1 and 2 who do not present electrocardiographic changes or enzyme elevation could be discharged with a low probability of cardiac events at 6 months. Whereas in patients with TIMI>3, hospitalization and treatment in line with current guidelines should be considered.
Dr Gimeno's contribution to this project was financed by a research grant from Merk Sharp and Dhome.
Correspondence: Dr. M. Valdés Chávarri.
Servicio de Cardiología. Hospital Universitario Virgen de la Arrixaca.
Ctra. Murcia-Cartagena, s/n. 30120 El Palmar. Murcia. España.
E-mail: valdeschavarri@valdeschavarri.e.telefonica.net