The present article reviews the most important publications and studies in the field of interventional cardiology in 2013. Coronary interventions for ST-segment elevation myocardial infarction are among the most important, with studies that assess different devices and pharmacologic and mechanical strategies in primary angioplasty. Increasingly large groups of patients (with diabetes, of advanced age) and the best coronary revascularization strategy are also the focus of exhaustive research. Percutaneous procedures in the left main coronary artery continue to give rise to a significant number of publications, both because of the results of using different types of stent and because of the intravascular imaging techniques used to guide procedures and the results of their use. New bioabsorbable polymer-coated drug-eluting stents or bioresorbable drug-eluting scaffolds are being compared with second-generation drug-eluting stents to show their efficacy in preventing restenosis and reducing incidence of late thrombosis. Percutaneous treatment of structural heart disease continues to produce many publications, especially regarding percutaneous aortic prostheses, but also on closure of foramen ovale and of left atrial appendage. Finally, renal denervation continues to arouse much interest in the medical literature.
Keywords
The STREAM study tried to clarify whether prehospital fibrinolysis within 3h of the onset of ST-segment elevation myocardial infarction (STEMI), combined with rapid coronary angiography, provides clinical results similar to those of primary elective percutaneous coronary intervention (PCI) when the latter may be substantially delayed. The authors randomized 1892 patients with STEMI to primary PCI or fibrinolytic treatment with tenecteplase (half-dose for those aged ≥ 75 years or over), clopidogrel and enoxaparin prior to transfer to a hospital equipped to perform PCI. If fibrinolysis failed, urgent coronary angiography was performed for rescue PCI. Otherwise, angiography was performed at 6h to 24h after randomization. No significant differences were found in the primary endpoint (composite of death, shock, congestive heart failure, and reinfarction up to 30 days). However, 36% of patients in the fibrinolysis group needed urgent angiography and incidence of intracranial bleeding was higher in the fibrinolysis group than in the primary PCI group.1–3
The TASTE study4 investigated whether thrombus aspiration reduces mortality in patients with STEMI. It randomized 7244 patients treated with primary PCI. No significant differences were found in primary endpoint (all-cause mortality at 30 days), although there was a nonsignificant trend towards more reinfarctions and stent thrombosis in the subgroup without thrombus aspiration.
The PRAMI study5 investigated the results in patients with STEMI of preventive PCI in the arteries not responsible but with significant stenosis. The authors included 465 patients with STEMI and multivessel disease treated with primary PCI and randomized to preventive PCI or not. Subsequent PCI was only recommended in refractory angina with objective evidence of ischemia. The composite primary endpoint was cardiac death, nonfatal myocardial infarction, or refractory angina. This study was stopped early, after a mean 23-month follow-up, on finding a significant reduction in the primary endpoint in the group of patients treated with preventive PCI.
In Spain, the prospective, multicenter, METOCARD-CNIC study assessed the benefit of an intravenous beta blocker (metoprolol) prior to primary PCI in STEMI and within 6h of symptoms onset. The authors randomized 220 patients. The primary endpoint (infarction size recorded by magnetic resonance imaging) was significantly less frequent in the metoprolol group whereas left ventricular ejection fraction was significantly higher.6,7
The impact of door-to-balloon time on mortality in STEMI has been reviewed in a large-scale US registry including over 96000 patients treated with primary PCI between 2005 and 2009. Although door-to-balloon time fell significantly between the first and last years recorded, 30-day mortality did not change even after adjustment for risk. The authors concluded that additional measures were needed to successfully reduce mortality in this population.8
The management of patients with cardiogenic shock secondary to STEMI remains controversial. The German IABP-SHOCK II study randomized 600 patients in shock after early revascularization to intra-aortic counterpulsation balloon treatment or not. In 1-year follow-up, no differences were found between the 2 groups for mortality, repeat revascularization, aortocoronary surgery, or stroke. However, there was a trend towards higher incidence of reinfarction in patients undergoing counterpulsation balloon treatment. In multivariate analysis, the independent predictors of mortality were age, history of stroke, and baseline serum lactic acid. This study follows European Cardiology Society and American Heart Association/American College of Cardiology guidelines in avoiding the systematic use of counterpulsation balloon in these patients unless they needed to achieve hemodynamic stability for transfer to a center equipped for revascularization procedures.9,10
Non–ST-segment Elevation Myocardial InfarctionThe TAO study11 compared the efficacy and safety of otamixaban, an intravenous direct factor Xa inhibitor, with heparin plus eptifibatide in patients with non—ST-segment elevation myocardial infarction undergoing early invasive procedures. The study randomized 13 229 patients in 55 countries. The primary endpoint for efficacy was the composite of all-cause death or new myocardial infarction within 7 days. There were no significant differences between groups for efficacy, but the primary safety endpoint (more or less TIMI bleeding) increased significantly in parallel with otamixaban in all subgroups. Given these findings, otamixaban administration is not recommended in this context for safety reasons.
The ACCOAST study12 included over 4000 patients. It assessed prasugrel administration at diagnosis of non—ST-segment elevation myocardial infarction or immediately prior to PCI after coronary angiography. The safety committee halted the study early when increased severe bleeding was detected with no reduction in the principle efficacy endpoint (the composite of cardiac death, myocardial infarction, stroke, urgent revascularization, or rescue treatment with glycoprotein IIb/IIIa receptor inhibitors). They concluded that in patients with non—ST-segment elevation myocardial infarction in a catheterization program, prior treatment with prasugrel does not reduce the rate of ischemic events at 30 days, but does increase the rate of bleeding complications.
Patients With DiabetesThe controversy over percutaneous or surgical revascularization for patients with diabetes has given rise to numerous studies. The FREEDOM study included 1900 patients with diabetes and multivessel disease (excluding left main coronary artery lesion) who, between 2005 and 2010, were randomized to surgical or percutaneous revascularization with paclitaxel-eluting stents. After nearly 4 years follow-up, the composite primary endpoint of death, infarction, and stroke was significantly greater in the PCI group, whereas incidence of the need for new revascularization fell. The authors concluded that in patients with diabetes and multivessel coronary disease, surgical revascularization is better than PCI because it significantly reduces rates of death and infarction, even though it does produce a higher rate of stroke.13 This study analyzed the cost-effect ratio of PCI deploying drug-eluting stents (DES), compared to myocardial revascularization surgery. In patients with diabetes and multivessel disease, the initial costs were greater due to hospitalization and immediate complications. Total cost at 5 years after surgery was also greater but the cost of PCI follow-up was growing.14
Older PatientsThe XIMA study15 included 800 patients > 80 years (mean, 83), randomized to everolimus-eluting or bare metal stent. The primary composite endpoint of death, infarction, target vessel revascularization, stroke, and major bleeding showed a nonsignificant trend in favor of the everolimus-eluting stent, based particularly on lower incidence of stroke and revascularization in the follow-up.
Vascular AccessThe European Association of Percutaneous Cardiovascular Interventions has published a consensus document on radial access in percutaneous procedures. Twenty years after vascular access was first introduced in daily clinical practice, differences over its use remain among interventional cardiologists, hospitals, and countries. The document collates the numerous favorable references (especially on the reduction in vascular complications), defines the current role of radial access, and reviews the technique, learning process, organizational implications, and clinical indications.16
Coronary Disease: Specific LesionsLeft Coronary ArteryThe ESTROFA-LM registry17 is a multicenter, retrospective registry conducted in Spain. It included 770 patients with severe left main coronary artery disease treated consecutively with first- and second-generation DES (415 with paclitaxel and 355 with everolimus). In the 3-year follow-up, no significant differences were found in mortality, reinfarction, target vessel revascularization, or stent thrombosis. Deployment of two stents, age, diabetes mellitus, and acute coronary syndrome were independent predictors of mortality; use of intravascular ultrasound (IVUS) in the subgroup with distal involvement was an independent predictor of improved prognosis.
The DKCRUSH-III study18 compared the results of two double stenting techniques (double kissing crush stenting vs culotte stenting) in severe distal bifurcation lesions of the (unprotected) left main coronary artery. The study randomized 419 patients with a primary endpoint of cardiac event (death, infarction, or need for revascularization) at 1 year. Patients treated with the culotte technique showed a significantly higher incidence of cardiac events, whereas incidence of target vessel revascularization, and particularly secondary vessel restenosis, fell.
RestenosisIn Sweden, the SCAAR registry of angiography and angioplasty recorded all interventions in the country.19 The authors reviewed all restenosis procedures identified (7806 segments) between 2005 and 2012 and their treatment. For conventional stent re-restenosis, adjusted risk was significantly lower with DES, and tended to be lower with drug-eluting balloons; however, it was higher with a new conventional stent than with balloon angioplasty. For DES restenosis, a new DES was associated with a significant reduction in risk-adjusted re-restenosis and a similar but nonsignificant reduction compared to drug-eluting balloon or conventional stent compared with balloon angioplasty. A change of drug in the new DES was no more efficient than using the same drug. The authors concluded that a DES or a drug-eluting balloon should be used to treat restenosis of a conventional stent and that DES restenosis treatments produced higher re-restenosis rates than conventional stent re-restenosis.
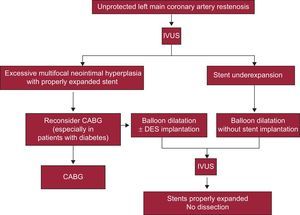
Restenosis of stents in the left main coronary artery constitutes an increasingly frequent problem due to the increased use of PCI in this segment. Although many patients can be referred for surgery, others have previously had surgical revascularization ruled out. De Caterina et al20 reviewed the incidence, predictors, and current management of this entity. The studies indicate that left main coronary artery restenosis is the principle predictor of clinical events following PCI. However, information about management of this context is scarce. An algorithm has been proposed that includes IVUS as an essential component to rule out hyperplasia, stent underexpansion, or less frequent problems like stent fracture and, according to these findings, to direct patients towards surgery, stent reimplantation, or balloon angioplasty (Figure 1).
Algorithm for restenosis management in the left main coronary artery. CABG, coronary artery bypass grafting; DES, drug-eluting stent; IVUS, intravascular ultrasound. Reproduced with permission from De Caterina et al.20
In Spain, the CIBELES trial21 randomized patients with chronic coronary occlusions and indication for revascularization to PCI with first- (sirolimus) or second-generation (everolimus) DES. The trial included 207 patients with a primary angiographic endpoint of late luminal loss in the stent at 9 months and clinical follow-up at 1 year. No significant angiographic differences were found between the two stent types in the rate of binary angiographic restenosis or that of clinical events at 1-year follow-up. The rate of stent thrombosis showed a nonsignificant unfavorable trend with the sirolimus-eluting stent. The authors concluded that both DES types are equally efficient in the revascularization of chronic coronary occlusions.
Drug-eluting StentsThe NEXT study22 is a randomized multicenter trial that compared efficacy and safety in daily clinical practice (with no exclusion criteria) of a biodegradable polymer biolimus-eluting stent with a permanent polymer everolimus-eluting stent. The study included 3200 patients with a primary endpoint for efficacy at 1 year (target lesion revascularization) and a primary endpoint for safety at 3 years (death or infarction). The results showed no differences in the primary efficacy endpoint at 1 year, late luminal loss in the angiographic substudy, and the safety endpoint at 3 years, with very low rates of thrombosis in both stent types.
The BIOFLOW-II study23 compared a new sirolimus-eluting stent with a biodegradable polymer vs an everolimus-eluting stent with a permanent polymer. The study included 425 patients, randomized 2:1, with a primary endpoint of late luminal loss that showed noninferiority of the new stent. The secondary clinical endpoints showed no significant differences with respect to target vessel revascularization, infarction, thrombosis, or death. Intravascular imaging confirmed less intimal hyperplasia with the new stent at 9 months.
Bioabsorbable Stents (Bioabsorbable Vascular Scaffold)The new platforms of totally biodegradable stents are bound to give rise to numerous studies in the coming years. Currently, the Absorb BVS (bioabsorbable vascular scaffold) is the only commercially available device and its indications for use are being gradually broadened.
The safety of BVS implantation in patients with acute coronary syndrome has been studied in 150 consecutive patients (194 lesions) vs a control group of 103 patients (129 lesions) treated with an everolimus-eluting stent. Incidence of major cardiac events was similar in both groups, although in 2 cases the BVS could not be delivered to the lesion. In multivariate analysis, BVS use did not correlate with the appearance of adverse cardiac events.24,25
The PRAGUE-19 registry26 has assessed the safety and efficacy of this device in primary PCI in patients with STEMI. A BVS device was implanted in 22 patients with no complications other than in cases where current device dimensions did not adapt adequately to the size of the artery and in arteries with calcified lesions or severe tortuosity.
Percutaneous Treatment of Heart Disease: Antiplatelet and Anticoagulation TherapyPatient response to antiplatelet drugs varies enormously. Moreover, we do not know whether monitoring platelet function to adjust antiplatelet treatment benefits patients who undergo PCI with DES. The ARCTIC trial27 included 2440 patients randomized to platelet function monitoring with the VerifyNow® system or a conventional nonmonitoring strategy. The primary endpoint was the composite of death, infarction, stent thrombosis, stroke, or urgent revascularization within 1 year of stent implantation. The trial showed no significant improvement in clinical results was achieved by monitoring platelet function and adjusting treatment for DES implantation, compared to routine antiplatelet agent treatment without monitoring.
The TRILOGY-ACS study28 included a substudy of platelet function that analyzed the differences between over 2500 patients after acute coronary syndrome who did not require revascularization and received medical treatment alone (clopidogrel or prasugrel plus acetylsalicylic acid). The principle parameter assessed was platelet reactivity and the primary endpoint of efficacy was the composite of cardiac death, infarction, and stroke at 30 months. Although prasugrel was associated with less platelet reactivity than clopidogrel-independently of age, weight, and dose-no significant differences were found between prasugrel and clopidogrel in terms of frequency of the primary endpoint for efficacy at 30 months; nor was any significant association found between platelet reactivity and the appearance of ischemic events.
The CHAMPION PHOENIX trial29 assessed the role of cangre-lor—a powerful, rapid-acting, antiplatelet, adenosine diphosphate receptor antagonist used intravenously—in over 11 000 patients undergoing PCI and randomized to bolus and cangrelor infusion or loading dose of clopidogrel. The primary endpoint of efficacy was the composite of death, infarction, revascularization for ischemia, or stent thrombosis within 48h of randomization. The primary endpoint for safety was severe bleeding within 48h. The results show that cangrelor significantly reduces the rate of ischemic events during PCI, including stent thrombosis, with no significant increase in severe bleeding.
The PARIS registry30 is a prospective, observational study of patients undergoing PCI with stent implantation that assessed the different modes of dual antiplatelet therapy cessation and their association with risk following PCI. The registry included over 5000 patients and defined prespecified categories of dual antiplatelet therapy cessation: physician-recommended discontinuation, brief interruption (for surgery), or disruption (noncompliance or because of bleeding). The primary endpoint was the composite of cardiac death, stent thrombosis, infarction, or target lesion revascularization at 2 years; this was present in 11.5% of cases, most of which (74%) occurred while patients were receiving dual antiplatelet therapy. Although most events following PCI were in patients receiving dual antiplatelet therapy, early risk of an event is substantial, independently of stent type.
The Swedish SCAAR registry analyzed the results of administering heparin or bivalirudin to patients with non—ST-segment elevation myocardial infarction undergoing percutaneous revascularization between 2005 and 2011. Patients receiving glycoprotein IIb/IIIa inhibitors were excluded. After propensity score analysis, over 41 000 patients were included. The primary endpoint for efficacy was mortality at 30 days, which proved similar in both groups. However, multivariate analysis showed the adjusted odds ratio favored the group receiving heparin.31 These results have given rise to a randomized study comparing heparin with bivalirudin in patients with non—ST-segment elevation myocardial infarction and pretreated with the new adenosine diphosphate inhibitors.
Intracoronary Diagnostic TechniquesThe FIRST study32,33 is a prospective international registry of patients with intermediate coronary lesions (angiographic stenosis between 40% and 80%). The study's objective was to determine the correlation between minimal lumen area, measured by IVUS, and fractional flow reserve measured with pressure wire. The authors included 350 patients (367 lesions) and concluded that a minimal lumen area < 3.07 mm2 had a moderate correlation with an fractional flow reserve < 0.8 (sensitivity, 64%; specificity, 65%; area below the curve, 0.65), although precision improved after adjustment for reference vessel diameter. The fractional flow reserve correlated with plaque burden but not with other morphologic characteristics.
A retrospective registry34 has assessed the use of fractional flow reserve by comparison with coronary angiography prior to surgical revascularization in 627 patients with intermediate lesions. In the group of patients with fractional flow reserve-guided revascularization procedures, the number of needed grafts decreased, as did the percentage of interventions requiring extracorporeal circulation, with no repercussions on incidence of adverse events during the 3-year follow-up.
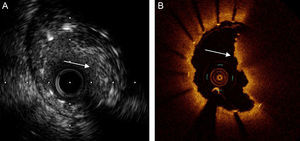
In Spain, a multicenter registry35 has included more than 1600 patients with DES deployment in the left main coronary artery. In more than 500, IVUS was used and a group of patients was selected in whom propensity score-matched IVUS was not used. The IVUS significantly reduced the composite of cardiac death and infarction at 3 years and stent thrombosis (Figure 2), especially in lesions distal to the left main coronary artery and in patients receiving 2 stents.
Percutaneous Treatment of Structural Heart DiseasePercutaneous Aortic Valve ImplantationThe results of the PARTNER 1 study36 showed a reduction in mortality at 1 year in the group undergoing percutaneous treatment. However, incidence of stroke and paravalvular regurgitation increased. Clinical follow-up at 3 years showed almost identical rates of mortality and stroke in both groups.
The SOURCE XT registry included more than 2600 patients treated with a percutaneous SAPIEN XT valve as standard clinical practice in 17 countries. In 1-year follow-up, total mortality was 19.5% (10.8%, of cardiac origin) and the rate for stroke was 6.3%. Moderate or severe paravalvular regurgitation was found in 6.7% of patients. Transfemoral access was associated with better survival rates than transapical or transaortic access; total mortality was significantly greater in women than in men.37
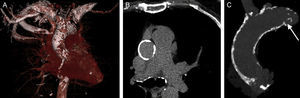
In Spain, a multicenter registry assessed the efficacy and safety of percutaneous implantation of a self-expanding valve in patients with severe aortic stenosis who had previously been rejected for valve replacement surgery because of a porcelain aorta. The study compared these patients with others receiving the same valve but without porcelain aorta (Figure 3). Clinical results were similar in both groups and the appearance of complications during implantation was the only predictor of the primary outcome (mortality at 2 years). The Editorial that accompanies this article proposes that percutaneous aortic valve implantation should be the standard treatment for patients with porcelain aorta.38,39
Multislice computerized tomography image showing a porcelain aorta. Highly calcified ascending thoracic aorta (A), circular in appearance (B) and with protruding plaque (C, arrow). Reproduced with permission from Van Mieghem and Van der Boon.39
The randomized RESPECT study40 prospectively assessed the efficacy of percutaneous closure of the patent foramen ovale in preventing recurrent stroke in patients with a history of cryptogenic stroke by comparing this with medical treatment. The study included 980 patients and no differences in primary outcome were found between groups during follow-up. However, significant differences were found in some subgroups and these favored percutaneous closure (atrial septal aneurysm, substantial right-left shunt).
In Europe, a randomized, multicenter study compared the efficacy and safety of devices used for percutaneous closure of the patent foramen ovale. The study included 660 patients and 3 devices were used (Amplatzer®, CardioSEAL-Starflex® and Helex®). The primary outcome was defined as the composite of recurrence of cerebral ischemia, death due to neurologic cause, or any paradoxic embolism within 5 years of the procedure. In the follow-up, the Amplatzer® showed a significantly lower rate of neurologic events than the CardioSEAL-Starflex® or Helex® devices.41
Percutaneous Closure of Left Atrial AppendageResults of the more than 2-year follow-up of patients included in the multicenter PROTECT AF study have been published.42 The authors included over 700 patients who were randomized 2:1 to device or oral anticoagulation therapy with warfarin. The primary outcome was the composite of stroke, systemic embolism, and cardiac death. After > 2-year mean follow-up, the device proved noninferior to oral anticoagulation.
Percutaneous Mitral Valve RepairThe EVEREST II study prospectively included 279 patients with severe mitral regurgitation, randomized 2:1 to treatment with the MitraClip® percutaneous system, repair surgery, or valve replacement. The primary outcome was the composite of survival, surgery for mitral valve dysfunction, or moderate-severe mitral regurgitation. At 1 year, the primary outcome favored surgery (essentially, over a substantial reduction in the need for intervention for valve dysfunction). However, at 4-year follow-up, the rates of mortality and moderate-severe mitral regurgitation balanced out, although the need for intervention for valve dysfunction was 5-fold greater in the percutaneous treatment group.43
Renal DenervationAfter 2-year follow-up, data from the multicenter, randomized, Symplicity HTN-2 study have been published. The study included more than 100 patients with resistant arterial hypertension. The significant fall in blood pressure seen at 6 and 12 months was maintained at 2 years, with no increase in cardiovascular events and no deterioration in renal function. Patients who crossed to the treatment arm also showed a similar significant reduction in blood pressure.44–46
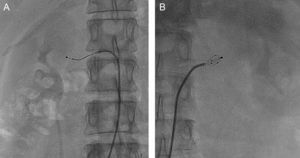
The EnligHTN I study's 1-year follow-up47 has reported on the use of a multielectrode catheter for renal denervation (Figure 4). The reduction in systolic and diastolic blood pressure was significant by comparison with the control group. This was maintained during the follow-up and no complications or cardiovascular events occurred.
Cell Therapy for Myocardial RegenerationIn this field, a meta-analysis has collated data from several randomized studies that analyze the impact of intracoronary therapy with bone marrow cells on left ventricular function following STEMI. The study included more than 1600 patients from 16 randomized studies. The intracoronary infusion of bone marrow cells associated with a significant increase in left ventricular function, especially in younger patients and in those with lower ejection fraction, without increasing complications.48
CONFLICTS OF INTERESTNone declared.