In the year 2012, 3 scientific sections—heart failure and transplant, congenital heart disease, and clinical cardiology—are presented together in the same article. The most relevant development in the area of heart failure and transplantation is the 2012 publication of the European guidelines for heart failure. These describe new possibilities for some drugs (eplerenone and ivabradine); expand the criteria for resynchronization, ventricular assist, and peritoneal dialysis; and cover possibilities of percutaneous repair of the mitral valve (MitraClip®). The survival of children with hypoplastic left heart syndrome in congenital heart diseases has improved significantly. Instructions for percutaneous techniques and devices have been revised and modified for the treatment of atrial septal defects, ostium secundum, and ventricular septal defects. Hybrid procedures for addressing structural congenital heart defects have become more widespread. In the area of clinical cardiology studies have demonstrated that percutaneous prosthesis implantation has lower mortality than surgical implantation. Use of the CHA2DS2-VASc criteria and of new anticoagulants (dabigatran, rivaroxaban and apixaban) is also recommended. In addition, the development of new sequencing techniques has enabled the analysis of multiple genes.
Keywords
.
DEVELOPMENTS IN CHRONIC HEART FAILUREThe most noteworthy recent development in heart failure (HF) in Europe has been the publication of the latest version of the clinical practice guidelines for the diagnosis and treatment of acute and chronic HF in 2012 by the European Society of Cardiology.1,2 The main novelties are presented in Table 1.
Main Updates in the Latest Version of the Clinical Practice Guidelines for the Diagnosis and Treatment Heart Failure
| • Presentation of the recommendations in tables that include the reference that supports the class of recommendation and level of evidence |
| • Specific and differentiated definition for diagnosis of heart failure with reduced ejection fraction and heart failure with preserved ejection fraction |
| • Extension of the indication of mineralocorticoid receptor antagonists (aldosterone antagonists) |
| • New indication for ivabradine |
| • More information on the role of coronary revascularization |
| • Extension of the indications for cardiac resynchronization therapy |
| • Recognition of the growing role of ventricular assist devices |
| • Reinforcement of the holistic management of patients and the role of multidisciplinary programs for the follow-up of heart failure |
The two most important pharmacological aspects of this new version are the inclusion in the guidelines for the first time of a specific recommendation for the use of ivabradine and the improvement in the level of evidence for the use of aldosterone antagonists.
The guidelines assign a class IIa recommendation, evidence level B, to the use of ivabradine on the basis of the SHIFT study results.3 Basically, this version now incorporates ivabradine into the therapeutic algorithm for patients already in treatment with diuretics if they need angiotensin converting enzyme inhibitors or angiotensin receptor antagonists, beta blockers, and aldosterone antagonists, provided that the patient is in sinus rhythm and has a heart rate>70 bpm. This drug is therefore indicated before cardiac resynchronization therapy should be considered. It should be mentioned that the cutoff point for starting this treatment according to these guidelines is 70 bpm, and not the slightly higher value of 75 bpm established by the European Medicines Agency.
Along the lines of the SHIFT study, several recently published substudies have added further interesting data to what was already available. Thus, a substudy published in the Journal of the American College of Cardiology shows that the benefit of ivabradine is more closely related to the baseline heart rate of the patient than to the dose of beta blockers.4 The baseline heart rate of the candidate for treatment with ivabradine seems to be a crucial factor: in one of the SHIFT substudies published in 2011, treatment with ivabradine showed improvement in important “hard” endpoints such as all-cause mortality and cardiovascular mortality in the cohort of patients with a baseline heart rate of 75 bpm or more, thereby highlighting that the higher the baseline heart rate the greater is the benefit of this drug.5
With the results of the EMPHASIS study,6 aldosterone antagonists (or mineralocorticoid receptor antagonists, as they are known in the latest version of the guidelines) now receive a class I recommendation with level of evidence A for symptomatic patients with HF and left ventricular ejection fraction (LVEF)≤35%. In addition to the evidence already available from previous studies such as RALES7 and EPHESUS8 (after acute myocardial infarction), the results of EMPHASIS study add a primary role in escalating pharmacological treatment after the introduction of beta blockers and before ivabradine in the recommended therapeutic algorithm in this version of the guidelines.1
With regard to previous guidelines, this new version shows little change in the indication for implantable cardioverter-defibrillator placement. Implantable cardioverter-defibrillator placement is recommended in primary prevention for patients in functional class II-IV with LVEF≤35%, but unlike previous guidelines, the patients should now have received at least 3 months of optimal therapy. In the case of cardiac resynchronization therapy, of note is the incorporation of patients with mild HF into the group of candidates in view of new studies incorporated into the latest version of the guidelines. Thus patients in New York Heart Association (NYHA) functional class II are now included. When establishing an exact recommendation class and given level of evidence, the current version lends much more weight to the functional class of the patient and the presence or absence of left-bundle-branch block. In all cases, a broad QRS complex is required (from 120ms to 150ms depending on the patient subgroup) and the presence of severe left ventricular dysfunction (LVEF≤35% for NYHA III-IV and LVEF≤30% for NYHA II). The recommendations are class I when left-bundle-branch block is present and class IIa for QRS morphologies.1
In the field of heart surgery and percutaneous valve procedures, the role of surgical revascularization is strengthened as a means to alter the natural disease course in patients with left-main stenosis or 3-vessel disease. Thus, surgical revascularization in patients with systolic dysfunction, angina, and 2- or 3-vessel disease (1 of which is the left anterior descending artery) with optimal conditions for surgery is a class I recommendation with level of evidence B. One development of note in the 2012 guidelines is that, for the first time, the option of percutaneous placement of an aortic valve prosthesis is considered in patients with a high surgical risk, and the possibility of edge-to-edge percutaneous valve repair (also known as the Alfieri percutaneous technique) is considered in patients with an indication for valve repair but who are considered inoperable or at an unacceptably high surgical risk. These guidelines do not recommend performing surgical repair of the left ventricle with coronary revascularization or using external containment devices in patients with left ventricular dilatation.1
Thus, this latest version sheds new light on many aspects of the management of HF. However, the new guidelines still leave much room for debate and point to many topics that require greater levels of evidence to support specific recommendations. Despite these outstanding areas under debate, and the lack of information in many fields, the guidelines are still useful for the daily management of patients with HF.
DEVELOPMENTS IN ADVANCED HEART FAILURE AND TRANSPLANTATIONThe 2012 European guidelines on HF recognize heart transplantation as the treatment of choice for certain patients, but merely list the indications and contraindications without any substantial changes with respect to the previous version.1 Also of note is the growing role of mechanical circulatory support due to the increased number of patients with advanced HF, the limited number of donor organs, and technical progress in ventricular assist devices. In particular, the following aspects are clarified:
- •
Nomenclature of ventricular assist according to function (bridge to decision, bridge to candidacy, bridge to transplantation, bridge to recovery, and destination therapy)
- •
Criteria for possible implantation of a ventricular assist device (according to ejection fraction, oxygen consumption, and clinical and hemodynamic criteria)
- •
Class of recommendation and levels of evidence for use of ventricular assist devices
Likewise, there is emphasis on the need to apply mechanical circulatory support before the patient experiences right ventricular dysfunction or multiorgan failure, as such an approach is clearly associated with better survival. The high cost and the complications inherent in the use of ventricular assist devices mean that their use is very limited. As a result, management of symptomatic patients with advanced HF is still a challenge for healthcare professionals.
On the other hand, continual renal replacement therapies have emerged as a feasible alternative to an exclusively pharmacology-based treatment.9 Continuous venovenous hemofiltration is generally used in acute fluid overload in oliguric patients,10,11 but logistic difficulties and high costs are associated with its use as maintenance therapy and the outcomes are only moderately good. Peritoneal dialysis (PD) consists of introducing an osmotic solution into the abdominal cavity; the peritoneum acts as a membrane that eliminates both the excess fluid and waste products. Two studies of PD published recently show promising results. In the study by Koch et al.,12 118 patients with severe refractory HF and renal failure underwent PD. Survival at 3, 6, and 12 months was 77% (95% confidence interval [95%CI], 70%-85%), 71% (95%CI, 62%-79%), and 55% (95%CI, 45%-64%), respectively.
Núñez et al.13 analyzed the efficacy of PD in patients with HF who met the following criteria: NYHA III-IV, congestive symptoms despite optimal therapy with ASA diuretics, 2 admissions for acute HF in recent months, and renal failure (glomerular filtration<60mL/min/1.73m2). The outcome measures were quality of life, 6-min walk test, NYHA functional class, and concentrations of brain natriuretic peptide, N-terminal pro-brain natriuretic peptide (NT-proBNP), and serum Ca-125 at 6 and 24 weeks, and days of hospitalization in the following 6 months. Of the 57 preselected patients, 25 were assigned to PD, and improvement was found in all parameters except serum markers.
The studies reinforce the results reported previously, in which 1-year and 2-year survival was 82% and 56%, respectively, and there was a decrease in the days of hospitalization after initiating PD.14
Notable progress in the treatment of advanced HF has also been made with advances in cardiac contractility modulation. This therapy consists of applying electrical impulses during the absolute ventricular refractory period of the contractile phase of the cardiac cycle, and in so doing, cardiac muscle contraction is enhanced (dP/dt). The device is activated regardless of whether asynchrony is present after 3 beats in sinus rhythm. It is therefore not useful for atrial fibrillation (AF) or in the presence of frequent ventricular ectopia.
Preclinical studies indicate that cardiac contractility modulation can normalize phosphorylation of key proteins and expression of genes that encode the proteins involved in calcium cycle regulation and contraction.15
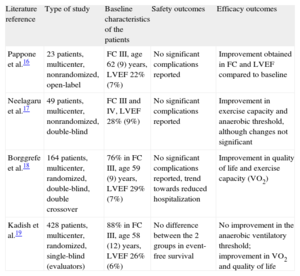
The device is given the European CE (Conformité Européenne in French) mark of compliance and is pending approval by the US Food and Drug Administration. To date, several trials have been performed in patients with severe ventricular dysfunction, advanced functional classes, narrow QRS complexes, and optimal therapy for HF (including defibrillator implantation), showing that patients who receive cardiac contractility modulation therapy have a better exercise capacity and quality of life compared to those without treatment (Table 2).16–19
Main Studies in Humans With Cardiac Contractility Modulation Therapy
| Literature reference | Type of study | Baseline characteristics of the patients | Safety outcomes | Efficacy outcomes |
| Pappone et al.16 | 23 patients, multicenter, nonrandomized, open-label | FC III, age 62 (9) years, LVEF 22% (7%) | No significant complications reported | Improvement obtained in FC and LVEF compared to baseline |
| Neelagaru et al.17 | 49 patients, multicenter, nonrandomized, double-blind | FC III and IV, LVEF 28% (9%) | No significant complications reported | Improvement in exercise capacity and anaerobic threshold, although changes not significant |
| Borggrefe et al.18 | 164 patients, multicenter, randomized, double-blind, double crossover | 76% in FC III, age 59 (9) years, LVEF 29% (7%) | No significant complications reported, trend towards reduced hospitalization | Improvement in quality of life and exercise capacity (VO2) |
| Kadish et al.19 | 428 patients, multicenter, randomized, single-blind (evaluators) | 88% in FC III, age 58 (12) years, LVEF 26% (6%) | No difference between the 2 groups in event-free survival | No improvement in the anaerobic ventilatory threshold; improvement in VO2 and quality of life |
FC, functional class; LVEF, left ventricular ejection fraction; VO2, oxygen consumption.
In recent years, the most important progress in congenital heart disease has come in the diagnosis, management, and improved outcomes of hypoplastic left heart syndrome. In the past 10 years, including the 3 palliative surgical procedures (Norwood stages I, II, and III), 5-year survival has improved from 50% to 69%, although the results vary greatly by hospital. In the last few months, numerous articles have been published on different aspects of the disease.
Prestage I. Diagnosis and prenatal management. Prenatal diagnosis of hypoplastic left heart syndrome is usually associated with a high rate of abortions, often because the healthcare professionals who counsel the parents are unaware of the actual prognosis of hypoplastic left heart syndrome. Hilton-Kamm et al.20 administered a questionnaire to 841 couples whose fetuses were diagnosed with severe congenital heart disease. The authors analyzed how counseling from the healthcare professionals on treatment and prognosis of hypoplastic left heart syndrome affected the final decision of the couple.
Stage I. This consists of reconstructing the aortic arch using the main pulmonary trunk. Coronary artery flow is achieved by retrograde filling of the hypoplastic ascending aorta, and creation of a nonrestrictive atrial septal defect. Pulmonary flow is achieved with a Gore-Tex conduit from the subclavian artery, Blalock-Taussig fistula, or flow from the right ventricle to the pulmonary arteries (Sano shunt). The advantages and drawbacks of one method or the other for establishing pulmonary flow has been the subject of much debate in recent years. Through histopathologic studies of samples obtained from autopsies or transplanted patients, Menon et al.21 found significant fibrosis in the area of the right ventriculotomy where the conduit was implanted in the Sano-type connection. Moreover, this corresponds to the area of depletion of the myocardial deformation, previously evidenced by echocardiography in the follow-up of one of the patients. On the other hand, Kolcz et al.22 reported that the Sano shunt ensured a more balanced flow distribution in both pulmonary branches and an improvement in the development of the left pulmonary artery. Finally, Frommelt et al.23 did not observe any difference in right ventricular size or function, or in the incidence of tricuspid valve regurgitation when the outcomes of the Blalock-Taussig fistula and the Sano shunt were compared at 14 months of age. Tabbutt et al.24 described the risk factors for major in-hospital morbidity and mortality after Norwood stage I intervention in a study that included 549 patients from 15 hospitals. A long cardiocirculatory arrest time with deep hypothermia, the need to keep the chest open after surgery, low weight, associated genetic abnormalities, need for postoperative extracorporeal membrane oxygenation, and hospitals whose surgeons had fewer patients were factors associated with a greater postoperative morbidity and mortality. However, there was no effect according to whether a Blalock-Taussig fistula or a Sano shunt procedure was performed. The hybrid procedure (stent implantation in the ductus arteriosus and introduction of bilateral branch pulmonary artery bands with surgical access and without extracorporeal circulation) has become an alternative in some hospitals in recent years. Despite high expectations, there do not appear to be any significant advantages in terms of overall survival of these patients after stage II, as reported by Photiadis et al.25
Stage II. In stage II, the superior vena cava is anastomosed with the pulmonary arteries (Glenn procedure) with no prior shunt. Menon et al.26 analyzed the risks of morbidity and mortality in a population of 162 patients from 31 hospitals in the United States. Low weight for their age, female sex, and aortic atresia with mitral atresia were associated with a higher incidence of mortality and complications.
Stage III. In stage III, the superior vena cava is anastomosed with the pulmonary arteries (Fontan procedure). Adequate systemic right ventricular function is essential for good postoperative outcomes in patients who undergo Fontan surgery; resynchronization is being incorporated with success for patients with a single ventricle, including hypoplastic left heart syndrome, to optimize myocardial contractility prior to the procedure, as described by Enomoto et al.27
Percutaneous and Hybrid Procedures in Congenital Heart DiseasePercutaneous closure of the ostium secundum with an Amplatzer® device is a safe and well-established procedure, although there may be early and long-term complications. The worst of these is arterial wall perforation resulting from device erosion. In a recent review by St. Jude Medical of perforation cases, 88% had a deficient aortic border (anterosuperior border in echocardiography) and the erosion causing the perforation occurred at this site. According to these data, the manufacturer has recently changed the recommendations concerning the contradictions in their use.28 These changes principally affect the assessment of the aortic border by echocardiography. Mallula et al.29 performed a systematic review of all possible sequential views with transesophageal echocardiography and intrathoracic echocardiography to study ostium secundum atrial septal defects and hypothesized that there is a very good correlation between transesophageal echocardiography and intrathoracic echocardiography for all borders and especially for the aortic border. The authors conclude that only a deficient aortic border in multiple sequential views, whether using transesophageal echocardiography or intrathoracic echocardiography, would be a contraindication for percutaneous closure of atrial septal defect because this procedure would be considered high risk in such cases.
Percutaneous closure of perimembranous and muscular ventricular septal defects with different specific devices has been the subject of several publications in recent years. Koneti et al.30 described a new percutaneous closure technique for ventricular septal defect by retrograde access and off-label use of the new Amplatzer® Duct Occluder II device. This was a prospective study that included 13 children with different ventricular septal defects, selected because they had isolated defects that were hemodynamically significant and measured <6.5mm in the echocardiography study (the maximum diameter available for the central cylinder of the Amplatzer® Duct Occluder II device is 6mm). The device was successfully deployed in 11 patients. The authors concluded that this technique was simple and significantly reduced fluoroscopy times.
Percutaneous approach to left ventricular outflow tract obstruction in symptomatic patients with obstructive hypertrophic cardiomyopathy (septal ablation with alcohol), which has long been a therapeutic alternative in adults, cannot be performed in children for technical reasons. Sreeram et al.31 published a study with preliminary results of percutaneous treatment of septal reduction by radiofrequency ablation (RFA) in 32 pediatric patients (2.9 to 7.5 years) with this disease. Most of the patients immediately showed a decrease in the left ventricular outflow tract gradient (78.5 [26.2] mmHg before RFA vs 36.1 [16.5] mmHg afterwards). The authors concluded that RFA is a therapeutic alternative for symptomatic pediatric patients with left ventricular outflow tract obstruction. The benefits of this procedure include ease of application, use of standard ablation equipment, and decreased hospital stay. The procedure can also be repeated in the same patient. The procedure is much safer than alcohol septal ablation and the ability to identify the His bundle and the possibility of preserving atrioventricular conduction are additional potential benefits of this technique.
The hybrid approach is one of the current challenges in the treatment of congenital structural heart defects, and requires close collaboration between surgeons and cardiologists to optimize the therapeutic strategy in each situation. Li et al.32 reviewed their 5-year experience in the hybrid approach with good outcomes in 30 patients with pulmonary atresia and an intact septum. They describe their algorithm for a subset of this population, for which right ventricular decompression is performed. Their strategy consists of an approach via a medial sternotomy, followed by right ventricular outflow tract perforation with an ultrasound-guided catheter and introduction of a guidewire into the pulmonary artery. The valve replacement procedure is then performed. In most cases, a modified Blalock-Taussig fistula is performed after ductus ligation. The authors conclude that this hybrid approach is safe, effective, and has theoretical advantages over conventional surgical or percutaneous treatment of this condition.
In a multicenter study,33 the experience from 11 centers in Germany, Austria, and Switzerland were reviewed for feasibility, efficacy, and outcomes of hybrid procedures for the closure of ventricular septal defects when the extracorporeal circulation time was minimized or completely eliminated. Twenty-six young patients with complex defects, including defects caused by myocardial infarction, were selected. In all cases, the surgical risk was high. Periventricular access was used in 20 patients, while in the remaining 6 patients, the occluders were placed with direct visualization as part of complex heart surgery. The device was correctly deployed in 23 patients (88.5%). The authors concluded that periventricular and intraoperative closure of ventricular septal defects with occluders is as effective as a surgical patch and that it avoids the increased morbidity of conventional surgical repair in a high-risk subgroup of patients.
VALVE DISEASE UPDATEIn 2011, progress in this field focused on percutaneous treatment of valve disease. The most important study was the arm of the PARTNER study in which it was shown that implantation of the Edwards-SAPIEN® prosthesis was not inferior to surgical replacement in high-risk surgical patients with severe aortic stenosis at 1 year.34 At 30 days, the all-cause mortality rates were 3.4% for transcatheter aortic valve implantation and 6.5% for surgery (P=.07), and 24% vs 26.8% at 1 year (P=.44). Strokes were reported in 3.8% who underwent transcatheter aortic valve implantation and in 2.1% who underwent surgery at 30 days (P=.07), and in 5.1% vs 2.4% in 1 year (P=.07). Symptoms improved markedly in both patient groups, and no significant differences were observed, although the hospital stay was shorter in the transcatheter aortic valve implantation group.
Of note regarding the pathophysiology of the aortic stenosis is a study using positron emission tomography that demonstrates the presence of inflammation in stenotic valves compared to healthy control valves.35 It is particularly interesting that this inflammation is increased in valves with mild and moderate stenosis, but not in those with severe stenosis, an observation that could lead to treatments that prevent aortic stenosis progression in early stages. A small multicenter Spanish study showed that axillary access in patients selected for transcatheter aortic valve implantation with CoreValve® and contraindication for femoral access is safe and effective and leads to excellent outcomes in terms of successful deployment and in-hospital mortality at 30 days.36 Another study of particular interest is a retrospective analysis of patients with a bicuspid valve, in which the incidence of dissection during a follow-up of 16 years is low (3.1 cases/10 000 patient-years), but significantly higher than that of the general population (relative risk [RR], 8.4; 95%CI, 2.1-33.5; P=.003).37
In the case of mitral valve disease, the EVEREST II study randomized 279 patients with grade 3 or 4 mitral valve regurgitation to percutaneous repair with MitraClip® or conventional surgery. After 12 months of follow-up, the MitraClip® was less effective in reducing mitral valve regurgitation than surgery (55% vs 73%; P=.007), although the percutaneous procedure was safer after 1 month of follow-up (15% of patients experienced adverse events compared to 48%; P<.001); the improvement in outcomes was similar in both cases.38
The study by Gertz et al.39 found that AF, a common occurrence in clinical practice, is associated with functional mitral valve regurgitation. Ablation of AF and return to sinus rhythm was associated with an improvement in valve function.
The management of tricuspid valve disease is complex because the patients, particularly in Spain, tend to have prior surgery, and so their surgical risk increases when they become symptomatic. Thus, the initial experience, in 14 cases, with percutaneous implantation of the Melody® pulmonary prosthesis in the tricuspid valve opens up new therapeutic options.40
Computed tomography also can be an excellent technique for discarding coronary lesions before valve surgery, making it unnecessary to perform an invasive study, provided the study is performed appropriately and the findings are negative.41
DEVELOPMENTS IN ELECTROCARDIOGRAPHYIn 2011, a study was published of 506 consecutive patients with chest pain attended in 55 peripheral health centers without a cardiologist on the staff. The patients were assessed by transtelephonic electrocardiogram (ECG) monitoring and analysis in a central facility. The patients were classified into 2 groups: A, patients without ECG abnormalities (n=445) and B, patients with abnormalities indicative of a cardiac etiology of the chest pain (n=61). The presence of risk factors was assessed by a multivariate analysis, which had high specificity (99.6%). Follow-up of the 506 patients confirmed the noncardiac origin of chest pain in 432 patients (97%) in group A and the cardiac origin in 59 (97%) of those in group B.42
To asses ECG abnormalities and coronary risk, a study of 2192 adults without prior heart disease was performed. ECG abnormalities were assessed at baseline and after 4 years of follow-up according to the Minnesota code. The primary outcome measure was a composite of coronary events (CEs). At the start of the study, minor ECG abnormalities were found in 13% while 23% presented with major abnormalities. During follow-up, 315 patients had a CE. The baseline ECG abnormalities were associated with an increase in the risk of a CE, which enabled a reclassification of the coronary risk in these patients. Thus, 176 intermediate-risk patients were reclassified as high-risk patients. After 4 years of follow-up, 208 patients had new ECG abnormalities and 416 had persistent abnormalities; in both cases, the presence of abnormalities was associated with an increased risk of a CE. This study provides evidence that ECG abnormalities can improve the prediction of coronary risk in elderly patients.43
Atrial FibrillationThe European guidelines for AF published in 2010 recommended the use of CHA2DS2-VASc criteria to calculate the risk of embolism in patients with nonvalvular AF and the use of new oral anti-platelet agents (OAPAs), which showed similar or better outcomes than warfarin in the prevention of stroke. Two new studies published in the past year provide new information about these drugs, although the findings had already been partially communicated elsewhere. The ARISTOTLE study included 18 201 patients (CHADS2 close to 2). The results showed that apixaban 5mg/12h was superior to warfarin in the prevention of stroke and systemic thromboembolism (RR=0.79; 95%CI, 0.66-0.95; P<.001), caused fewer serious bleeding events (RR=0.69; 95%CI, 0.60-0.80; P<.001), and reduced all-cause mortality (RR=0.89; 95%CI, 0.80-0.99; P<.001).44
The ROCKET study included 14 264 patients with a mean CHADS2 risk of 3. In the per-protocol analysis, rivaroxaban (20 mg/24 h) was not shown to be superior to warfarin in preventing embolic events and the incidence of serious or clinically relevant bleeding events. Compared to warfarin, rivaroxaban reduced the incidence of intracranial hemorrhage (0.5% vs 0.7%; P=.02) and fatal bleeding (0.2% vs 0.5%; P=.003). The 2 studies, along with the RE-LY study and its substudies, are solid evidence for the benefits of the new OAPAs.45
In the last few months, several groups have published data that support the usefulness of the CHA2DS2-VASc scale. Most have confirmed that there is still a significant difference between the recommendations for anticoagulation in the guidelines and clinical practice, and anticoagulation follow-up with international normalized ratio shows how difficult it is to maintain sufficient control.46–48 One of the criticisms of the new OAPAs is that their effects cannot be readily reversed, though studies are beginning to provide solutions to this issue.49
In 2012, the European Society of Cardiology published the updated guidelines for AF, which incorporate a review of these recent studies and recommend the use of the CHA2DS2-VASc and the new OAPAs dabigatran, rivaroxaban, and apixaban before warfarin.50
DEVELOPMENTS IN PRIMARY CAREOf note is a prospective study in 102 Spanish primary care (PC) centers that assessed therapeutic compliance with renin-angiotensin system inhibitors in 808 patients with uncontrolled hypertension and high vascular risk. The mean rate of properly taken doses was 87.9%. In general, 73.3% were adherent, 52.8% taking a dose a day and 46.5% at the correct time. Adherence was associated with fewer other concomitant medications and not having diabetes.51
As PC physicians have limited access to echocardiography in Spain, natriuretic peptides could be an alternative for diagnosis of HF. In a study performed in PC, the cut-off for NT-proBNP was determined in patients with suspected HF. An echocardiogram was recorded and assessed by a cardiologist. Of the 220 patients included, 65.5% were women and the median age was 74 years. Diagnosis of HF was confirmed in 52 patients (23.6%). The NT-proBNP values were 715 and 77.5 pg/mL in patients with and without HF, respectively. The optimal cut-off was 280 pg/mL (area under the receiver operating characteristic curve=0.94), that is, less than the one proposed in the NICE guidelines. Use of NT-proBNP in the clinic would have avoided 67% of the echocardiograms requested, and so it could be considered cost-effective.52
Peripheral arterial disease is considered as equivalent to the risk of ischemic heart disease, and so it is recommended to detect subclinical forms using the ankle-brachial index. A Spanish study analyzed the prevalence of peripheral arterial disease and the associated risk factors in a randomly selected sample of 2833 subjects. The prevalence of peripheral arterial disease (ankle-brachial index<0.9) was 3.7% (5.0% in men and 2.6% in women; P=.001). The current screening recommendations failed to detect 29.6% of the individuals with asymptomatic disease. The use of the ankle-brachial index increased by 32.7% the number of individuals identified with high coronary risk.53
There are still large gaps in our knowledge of the efficacy of blood-glucose control in the prevention of cardiovascular events. The ORIGIN study analyzed the efficacy of early intervention in blood-glucose control for prevention of cardiovascular events. The study included 12 537 prediabetic patients and patients with type 2 diabetes mellitus and a high cardiovascular risk to assess the effect of glargine insulin and standard antidiabetic treatment and omega-3 acids compared to placebo in the prevention of cardiovascular events. After follow-up of 6.2 years, the results showed a neutral impact in the group treated with glargine insulin and standard treatment for the primary outcome measure and for each of the components. A lower incidence of new cases of type 2 diabetes mellitus was observed (odds ratio=0.80; 95%CI, 0.64-1; P=.05). Weight gain and incidence of hypoglycemia were higher in the glargine insulin arm, although there were no differences in the incidence of cancer. The results for omega-3 acids were also neutral. The results of the ORIGIN study, in agreement with other studies, support the hypothesis that multifactorial intervention is the most efficient strategy.54
The responsibility for antiplatelet treatment for AF is shared between PC and the different specialties. The Val-FAAP study was designed to determine the characteristics of patients with AF attended in PC. A consecutive sample of 119 526 Spanish individuals was analyzed. The estimated prevalence of AF was 6.1% (n=3287), and this arrhythmia was associated with older age, hypertension, and male sex (P<.01). Permanent AF was the most frequent clinical form (45.3%) and this was associated with age and renal and heart disease. In 66% of the patients, AF presented with CHADS2≥2 although a third of these patients were not receiving antiplatelet therapy. In contrast, 48.8% of the patients with CHADS2=0 were receiving treatment. The data in this study show that AF is common in PC. It is important to provide information and training to enable better stratification and correct therapeutic management of these patients.55
DEVELOPMENTS IN HEREDITARY DISEASESOne article published in 2012 had a particular impact on our knowledge of the pathophysiology of right ventricular arrhythmogenic cardiomyopathy. This opened up a new line of investigation with regards to diagnostic tools and the future development of therapeutic agents. The group of Asimaki et al.,56 who had already reported an immunohistochemical technique with high sensitivity and specificity for diagnosis of arrhythmogenic cardiomyopathy from myocardial samples, has, through an unexpected finding, developed a pathophysiologic theory for this genetic disease. They performed a series of experiments with tissue and serum samples from patients with arrhythmogenic cardiomyopathy, sarcoidosis, and giant-cell myocarditis. The authors found certain parallels between these 2 types of myocarditis (highly arrhythmogenic) and arrhythmogenic cardiomyopathy itself. They demonstrated the effect of different inflammatory mediators such as interleukin-17, tumor necrosis factor alfa, and interleukin-6 in the translocation of plakoglobin (the main protein associated with the pathogenesis of arrhythmogenic cardiomyopathy) from the desmosome to the cytoplasm. These inflammatory factors were elevated in serum samples from patients with arrhythmogenic cardiomyopathy.
From the prognostic point of view, of note is a study of the risk of malignant ventricular arrhythmias in patients with dilated cardiomyopathy with lamin a/c mutations. The presence of moderate or severe systolic dysfunction (LVEF<45%), nonsustained ventricular tachycardia, male sex, and ins-del/truncating or mutations affecting splicing were independent markers of arrhythmic events. The presence of 2 factors was associated with a very high rate of events, and so ICD placement would be justified. This study is an example of the value of genetics, both in diagnosis of dilated cardiomyopathy and in risk stratification for sudden death. In this study, 18% of the patients had a major arrhythmic event within 4 years of follow-up. It is important to note that only 37% had LVEF<45% (mean, 55%). If the current recommendations (ACC/AHA/ESC guidelines) had been applied for ICD placement in patients with LVEF<35% in NYHA II-III, only 36% of the patients who suffered a major arrhythmic event would have been identified. A new era is dawning in which genetics will be able to identify subgroups of patients at high risk of arrhythmia who benefit from early invasive processes.57
An important technological advance in 2012 is the appearance in the clinical setting of massive ultrasequencing known as next generation sequencing. Until now, with the standard sequencing technique used, the Sanger method, it was only possible to analyze very short DNA fragments, often only 1 exon. Dozens of analyses had to be done to study just 1 gene, making the whole process slow and very expensive. With the next generation sequencing technique, it is possible to analyze hundreds of genes at the same time, or even the whole genome of each patient, with acceptable cost. Although this advance makes the sequencing process extremely quick, identification of multiple genetic variants of unknown meaning in the same patient also generates problems of interpretation. Currently, correct interpretation of the results is key in order to take advantage of the utility of this very powerful diagnostic tool. The discovery of new genes will necessarily go hand in hand with the development of animal models and functional studies to determine causality and the role of the new structural and functional proteins in the onset of hereditary heart disease. These and other aspects related to this new technique are reviewed at length by Ware et al.58
CONFLICTS OF INTERESTNone declared.