Keywords
INTRODUCTION
Ventricular preexcitation occurs when atrial electrical impulses reach (excite) the ventricles earlier than expected through a circuit other than the normal one that connects the atria with ventricles. Such a circuit is known as an accessory pathway or abnormal pathway, that is, a bundle or cardiac muscle tract that directly connects the atria with the ventricles. The phenomenon is often described as the Wolff-Parkinson-White (WPW) syndrome, though strictly speaking this term should only apply when ventricular preexcitation is associated with tachycardias. Nevertheless, many authors use the terms WPW syndrome and ventricular preexcitation synonymously, something which has often given rise to confusion in therapeutic recommendations.
When accessory pathways form a second circuit between the atria and ventricles, supraventricular tachycardias may arise because of a reentrant circuit in which the impulses travel through the atrium, across the atrioventricular node, through the His-Purkinje system, through the ventricle, through the accessory pathway and back to the atrium--the so-called tachycardias with circular impulse conduction. However, the most intriguing feature of these accessory pathways is that, because of their electrophysiological properties, they often transmit impulses more readily than the atrioventricular (AV) node, which acts as a physiological "filter" between the atria and ventricles. When a freely conducting AV accessory pathway (with atrial-to-ventricular conduction) is present, atrial tachyarrhythmias may lead to very fast and irregular ventricular rates which in turn may cause ventricular fibrillation. Moreover, atrial tachyarrhythmias, particularly fibrillation and atrial flutter, are more common in patients with ventricular preexcitation than in the general population. We are therefore faced with possibility of the coexistence of recurrent symptomatic paroxysmal supraventricular tachycardias that are never life-threatening and serious arrhythmias, with risk of sudden death. However, it is possible, in fact common according to population-based studies, that those with ventricular preexcitation do not have any type of arrhythmia and that the preexcitation is never detected because it does not cause symptoms in itself.
We should differentiate between this type of ventricular preexcitation and (fairly common) accessory pathways which may cause ventricular-to-atrial conduction, but not atrial-to-ventricular conduction, and so never cause ventricular preexcitation (normal electrocardiogram during sinus rhythm). These are the so-called hidden pathways, which may also cause paroxysmal tachycardias but which do not present a risk of serious arrhythmias or sudden death.
Preexcitation can be detected as a characteristic electrocardiographic abnormality (the so-called "delta wave" with a typically short PR interval), and so the condition is often found in those who undergo an electrocardiogram without having presented arrhythmias (prevalence of 1-3). We therefore need to be able to assess the possible risk to the individual and whether it is appropriate to perform a therapeutic intervention.
Treatment is administered in several clinical contexts: acute therapy for episodes of tachyarrhythmia, prevention of tachyarrhythmias and prevention of sudden death. Our main therapeutic alternatives are antiarrhythmic drugs and catheter ablation. The aim of the present review is to discuss the safety and effectiveness of these therapies in each context, in light of the broad but incomplete body of scientific literature and clinical recommendations published in recent years.1,2 We will concentrate in particular on catheter ablation, which has become extremely important since its widespread introduction at the beginning of the nineties. We will also pay particular attention to therapeutic strategy in asymptomatic individuals with ventricular preexcitation because of the greater uncertainty and anxiety for both the physician and the individual with preexcitation.
ACUTE TREATMENT OF TACHYARRYTHMIA EPISODES
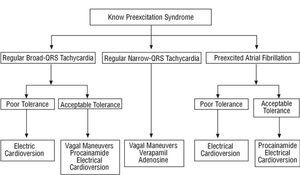
Regular Narrow-QRS Tachycardia (Figure 1)
The most common arrhythmia in patients with preexcitation is regular narrow-QRS tachycardia. The usual mechanism is orthodromic tachycardia, a variant of tachycardia with circular impulse conduction. Such tachycardias will usually respond extremely well to maneuvers with momentary nodal conduction block because the AV node is involved in the reentrant circuit. Thus, vagal maneuvers should be used initially (Valsalva maneuver or unilateral carotid sinus massage). If these fail, intravenous drugs should be administered. At the same time as any of these interventions, an electrocardiogram (in at least one lead) should be recorded on paper--watching the monitor is not sufficient because some findings can only be interpreted retrospectively (such as quantifying a pause, termination followed by reinitiation, etc). Adenosine (or adenosine triphosphate [ATP]) or nondihydropyridine calcium antagonists can be used, all of which have success rates of greater than 90%.3-5 All such drugs are safe except in certain situations. Adenosine should not be used if the patient suffers from bronchial asthma and calcium antagonists should be avoided in hypotensive patients, those with documented severe structural heart disease or those who are receiving beta-blockers.
Adenosine has a very short half life (less than 1 minute), and so it should be injected as a rapid bolus, and the tachycardia is terminated after 20-30 seconds. The patient should be warned that this treatment causes a sudden sensation of dyspnea and heat (and sometimes a "near-death experience"), extremely unpleasant but short-lived. The effect can be so brief that the tachycardia recurs a few seconds after termination. Atrial fibrillation--generally self-limiting and short-lived--may occur (in between 1% and 15% of the patients). If fibrillation persists, it will cause atrial fibrillation with preexcitation (see below).
Verapamil should be injected more slowly (5 mg in 2 min) and the effect may not develop until several minutes later. The dose is repeated every 5 minutes if the tachycardia continues, up to a maximum total dose of 15 mg. Carotid sinus massage should be repeated after every dose of verapamil because the drug sensitizes the patient to this maneuver, which may now be effective even though it was ineffective earlier.
Regular Broad-QRS Tachycardia (Figure 1)
Contrary to the general belief, regular broad-QRS tachycardia rarely occurs spontaneously in patients with ventricular preexcitation. All regular broad-QRS tachycardias are generally considered as ventricular unless proven otherwise, but such tachycardias are usually supraventricular in patients with known ventricular preexcitation. Different mechanisms may be responsible, for example, orthodromic tachycardia with bundle-branch block, antidromic tachycardia, atrial tachycardia or flutter with accessory pathway conduction and even preexcited nodal tachycardia.
If the patient shows poor clinical tolerance, electrical cardioversion should be performed; otherwise antiarrhythmic drugs can be tried. Vagal maneuvers should be used initially as they may terminate some of these tachycardias and are safe. Given that several of the mechanisms mentioned earlier do not involve the AV node and, in such cases, drugs that block the AV node may be harmful (see the following section), we believe it is safer to use drugs such as procainamide or intravenous flecainide2 that block accessory pathway conduction (the ventricle of these patients is usually undamaged). No specific studies have investigated use of adenosine in this situation, but this drug is unlikely to terminate or modify the tachycardia and might provoke atrial fibrillation, so we advise against its use. If a pharmacological treatment fails, the surest approach is to perform electrical cardioversion because combining intravenous antiarrhythmic drugs can be dangerous.
Preexcited Atrial Fibrillation (Figure 1)
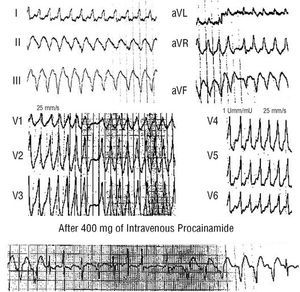
Preexcited atrial fibrillation presents with an electrocardiogram consisting of irregular, often rapid, tachycardia with broad QRS complexes of similar morphology (Figure 2) (unlike polymorphic ventricular tachycardias). Characteristically, one or several beats with a narrow QRS complex are intercalated.
Intravenous procainamide has usually been used provided tolerance was acceptable. This drug reduces ventricular frequency through blockade of the accessory pathway, leading to a greater number of narrow QRS complexes (Figure 2),6 and sinus rhythm may be recovered. Intravenous flecainide represents an alternative to procainamide. Nevertheless, if a pharmacological treatment fails, the best option is electrical cardioversion.
Some drugs used for treatment of episodes of atrial fibrillation can be harmful in patients with preexcitation for the following reasons. The drugs may directly reduce the refractory period of the accessory pathway, increasing ventricular frequency. Alternatively, there may be an indirect reduction in the refractory period of the accessory pathway, mediated in all likelihood by catecholamine release related to hypotension. AV node block may also arise, which is not beneficial and may be harmful because it also encourages preferential conduction through the accessory pathway. Finally, if no beneficial effects are obtained, the administration of a truly effective treatment may be delayed. Among the potentially harmful drugs, the best known are digoxin and verapamil.1,6 In addition, a series of patients were reported to suffer ventricular fibrillation after administration of intravenous amiodarone,7 so we think that this drug should also be contraindicated in preexcited atrial fibrillation.
It may be difficult to distinguish between atrial fibrillation with preexcitation and atrial fibrillation with bundle-branch block in a patient with irregular broad-QRS tachycardia and no previous indication of preexcitation. Adenosine may therefore be useful as this drug does not substantially affect the preexcitation ECG but does notably reduce ventricular response (for a few seconds) if the patient does not have preexcitation but bundle-branch block. Nevertheless, this maneuver should only be applied when a defibrillator is available to deal with possible ventricular fibrillation in preexcitation provoked by catecholamine release induced by adenosine (although such an eventuality has not been reported). If this is not possible, we think it best to resort to the therapeutic approach proposed in this article as if the patient had preexcited atrial fibrillation, even though the diagnosis of preexcitation is not definite. Electrical cardioversion should be used in cases of doubt, as the safest treatment.
PROPHYLACTIC DRUGS
There are no controlled studies that compare antiarrhythmic drugs in prophylaxis of arrhythmias (much less for prevention of sudden death) in patients with ventricular preexcitation.2 In fact, studies published with drugs such as propafenone, flecainide, sotalol, and amiodarone have been performed with very small patient populations, mainly with regular tachycardia. These studies showed good efficacy--greater than 70%--though follow up was short for a chronic process.8-11 Moreover, the side effects are not negligible with any of these antiarrhythmics, and may be particularly serious with amiodarone.
Currently, in view of the good results with radiofrequency catheter ablation, the opinion of the authors is that pharmacological treatment should be used only in patients in whom ablation has failed or who are not eligible due to problems of vascular access or poor clinical condition. Pharmacological treatment may also have to be used in those who refuse to undergo an invasive procedure.
CATHETER ABLATION
Certain radiofrequency energy pulses from catheters can induce small necrotic lesions (a few millimeters in diameter), a technique known as radiofrequency ablation. Given that the accessory pathways have small diameters, they can often be eliminated using this technique, provided the catheter is placed close to the accessory pathway in a sufficiently stable position. The cure is permanent because the lesions are irreversible. Since the first publications in 1991,12-15 the reported success rates have been so high that the technique has become the method of choice for treatment of WPW syndrome and comparative controlled studies with other treatments have been considered unnecessary (and unethical).
The success rate in all published studies exceeds 90%. In both an American multicenter study16 and the Spanish registries from 2001 and 2002,17,18 the acute success rate was 93%, with some variation according to site--the highest success rates being reported for the left lateral pathways. Once acute success has been achieved, the probability of recurrence is less than 5% and any recurrence will happen within the first 6 months.
In series published with a lower bias and with a large number of patients, a small but significant rate of complications can be discerned, some of which are serious with permanent sequelae and may, very occasionally, lead to death.16-20 Table 1 shows the data from the most important series published. This table only collates complications that are considered truly important, such as stroke, pericardial tamponade, myocardial infarction or complete atrioventricular block requiring a pacemaker. The mortality rate for this procedure approaches 1. Furthermore, it should be remembered that these complication rates are the best possible, both because some complications may not be reported to these registries or notification may be retrospective and because these studies tend to focus on hospitals with more experience. Although some technological advances may reduce the specific risk at some ablation sites,21 this is not expected to greatly lower complication rates in the near future.
RISK OF SUDDEN DEATH IN PREEXCITATION SYNDROMES
Population-Based Studies
Very long-term prospective studies were conducted in the second half of the twentieth century to assess the size of risk in young individuals in whom detection is straight forward with ECG but most of whom have few or no symptoms. These studies form the true basis of our knowledge of risk in individuals with preexcitation.
In 1968, Berkman and Lamb22 reported the clinical course of 128 men in the United States air force with preexcitation. Follow up lasted a minimum of 5 years and a maximum of 28 years, with a total follow up period of 2251 patient-years. At the start of follow up, only 13% of the patients had tachycardias. Two more patients developed tachycardias during the study period. Three deaths occurred, one in a flying accident in which the individual with preexcitation was not flying, another was a suicide and no information is available on the third.
Soria et al23 studied 270 people with preexcitation (from a population of 226 464 individuals corresponding to a prevalence 1.2). Of these, only 40% were asymptomatic. During a mean follow up of 5.7 years, there were 7 deaths--4 of noncardiac origin and 2 were sudden deaths.
Munger et al24 identified 113 subjects with preexcitation in an area of the state of Minnesota between 1953 and 1989. Of these subjects, 53% (60 patients) were asymptomatic--35 with documented arrhythmias--at the start of the study. During follow up of almost 12 years per patient, 2 sudden deaths occurred. This study was extremely meticulous and has been extensively cited as showing the risk of sudden death in asymptomatic individual with preexcitation. However, this is an incorrect interpretation of the study because the authors clearly established that the 2 patients who experienced sudden death belonged to the symptomatic group of subjects.24 None of the 53 symptomatic individuals followed for 537 patient-years died suddenly.
Two recent studies have enriched our information. A Greek group reported data from 157 subjects with preexcitation--77 of whom were asymptomatic--who were followed for a mean of 55 months.25 No sudden deaths were reported.
Fitzsimmons et al26 studied 238 military airmen with preexcitation over an extremely long period (mean, 11.7 years). Only 28 were symptomatic at the start of the study. There was only 1 sudden death during the study and, once again, this subject belonged to the group of symptomatic individuals. The authors reported that this patient had documented supraventricular tachycardia and atrial fibrillation before his sudden death.
In a recent well informed review, Todd et al27 also described a "special" type of population-based study performed in a small series of patients with preexcitation. Asymptomatic individuals underwent an electrophysiological study and were then followed to determine which electrophysiological parameters predict risk of sudden death or tachycardias.27 In these studies,28-33 follow up was shorter than in genuine population-based studies--between 1 and 7 years. A total of 257 patients were included in series of between 15 and 75 individuals. No sudden deaths were reported.
Two recent studies of this type published by the same group in prestigious journals have caused a great deal of scientific controversy and have once again brought the question of "risk" in asymptomatic preexcitation syndromes to the fore.34,35 In our opinion, the results of these studies are not so different from those published previously because of differences in the way the word "risk" is used. In these studies, "risk" refers not to sudden death or ventricular fibrillation but to any sustained tachycardia. This usage seems inappropriate to us because paroxysmal supraventricular tachycardias in themselves are not generally considered life-threatening. In the first of these studies, 212 asymptomatic individuals with preexcitation underwent an electrophysiological study. Their clinical course was then followed for a mean of 38 months, although the authors report that only 162 individuals "completed follow up."34 During the study, 33 individuals developed arrhythmic events--2 ventricular fibrillations and one sudden death. Once again, these 3 patients were among the 8 individuals who had developed atrial fibrillation previously but had refused to undergo ablation treatment. The authors highlight the predictive capacity of the electrophysiological assessment, as 29 of the 33 individuals who developed spontaneous arrhythmias were among the 47 with inducible arrhythmia. But the important point, in agreement with others studies, is that no patient suffered cardiac arrest or sudden death without having experienced prior arrhythmic events. A subsequent study by the same group randomly assigned 72 asymptomatic individuals with "high risk of some arrhythmic event," defined according to their previous study, to radiofrequency ablation or no treatment.35 As expected, 21 of 35 patients in the control group developed arrhythmias, a much higher proportion than that in the ablation group, but in 20 of these 21 patients, the arrhythmias were treated without sequelae or a risk to life. It should be noted, however, that a patient in the control group developed ventricular fibrillation and had to be revived.
All follow up periods of all asymptomatic individuals included in the population studies add up to a total of 6041 patient-years. During this time, 2 sudden deaths occurred which were not preceded by other arrhythmic manifestations so treatment was impossible. The rate of sudden death is 3.3 per 10 000 patient-years.
Hospital Studies
Some groups with a large number of case studies of electrophysiological or surgical assessment in patients with preexcitation have reported experience of small groups of patients with preexcitation and cardiac arrest due to ventricular fibrillation and have described the "profile" of these patients.
In an exceptionally clear and meticulous article, Klein et al36 compared 25 patients who had ventricular fibrillation in a preexcitation syndrome with 73 patients with this syndrome but without ventricular fibrillation. The authors highlight that ventricular fibrillation was the first symptomatic manifestation in only 3 of these 25 patients. The most frequent prior event was the presentation of both paroxysmal tachycardia and atrial fibrillation (14 of the 25 patients).36
In a population pooled from 7 European hospitals, 23 patients were found to have preexcitation and ventricular fibrillation. For 6 of these, these were the first symptomatic manifestation.37 The group in Maastricht reports that in 3 of the 12 cases of preexcitation and in 8 of the 15 cases of ventricular fibrillation, this was the first symptomatic manifestation (out of 690 patients with preexcitation studied).38,39
Taken overall, these studies corroborate that there is some risk of ventricular fibrillation in patients with ventricular preexcitation, confirm that ventricular fibrillation is more frequent if preceded by other arrhythmias; and favor the indication of ablation in symptomatic patients.
PREDICTION OF RISK
Some electrophysiological parameters, specifically, evaluation of electrophysiological properties of the accessory pathway such as refractory period, atrial frequency leading to 1-to-1 ventricular stimulation and, above all, ventricular response during induced atrial fibrillation (expressed as minimum and mean RR) are significantly different for patients who have presented with ventricular fibrillation compared to those who have not.31,33,36 This used to be particularly relevant, especially when the only nonpharmacological therapeutic option was surgery, with the consequent risk. However, in the era of catheter ablation--a low risk procedure--the equation is rather different.
A substantial proportion, between 30% and 50% of asymptomatic individuals evaluated electrophysiologically, present "high-risk" parameters, but epidemiological studies affirm that their risk (to life, not of tachycardias) is minimal, as we have just seen. Even in the study by Pappone et al,34 only 3 of 47 patients with inducible tachycardias showed ventricular fibrillation (and, as has been stated, all presented previous nonfatal tachyarrhythmias). Thus, for detecting risk to life, which is what is important in this situation, the specificity of the electrophysiological evaluation is minimal. This point of view has been defended by some experts for several years.40
Even less clear is the usefulness of "noninvasive" assessment to stratify risk. Information has always been based on correlations between a noninvasive test and electrophysiological assessment.41-44 Findings such as the disappearance of preexcitation after infusion of procainamide or ajmaline during exercise testing or, simpler still, the presence of intermittent preexcitation (which may even have extreme manifestations45) have been correlated with "low-risk" electrophysiological findings. Such observations may be comforting but their value has never been evaluated in a prospective epidemiological study--studies have been limited to comparison of surrogates and so, faced with this lack of precision, we cannot attribute much value to these stratifications. Once again, those patients who do not present the stratifying variable are also at low risk.
Although ambulatory monitoring with a Holter device is not usually considered for a patient with preexcitation, the so-called "cardiac events monitor" can play a role in determining whether a patient with palpitations that may be caused by arrhythmias has pathological tachycardias.46
THERAPEUTIC STRATEGIES
Figure 1 proposes an acute treatment strategy for tachycardias in a patient with known preexcitation. This strategy basically coincides with that of guidelines for clinical practice,1,2,6 but with small modifications because in this review we assume that the patient has preexcitation. For this reason, vagal maneuvers in broad-QRS tachycardias may be useful. Of particular note is that intravenous amiodarone, although overused in Spain, does not figure either in our strategy or in the published guidelines, including the guidelines for atrial fibrillation.6
Fig. 1. Acute treatment strategy for tachyarrhythmias in patients with known preexcitation syndrome. Drug administration routes are intravenous.
Fig. 2. Electrocardiogram of atrial fibrillation in a patient with preexcitation syndrome. The tachycardia is irregular and rapid with a broad QRS complex. Note all QRS complexes have essentially the same shape, unlike polymorphic ventricular tachycardias, which are also rapid and irregular but with beat-to-beat variations in the QRS complexes in each lead. One beat (asterisk) is narrower, probably because of simultaneous conduction by the normal conduction system and the accessory pathway (fusion complex). A concordant shape in the precordial leads can be seen (dominant R waves in all leads), which is very characteristic of the preexcited complex of some lateral left accessory pathways. The lower panel shows the ventricular response decreasing and giving way to the appearance of narrower and less preexcited QRS complexes during intravenous procainamide infusion.
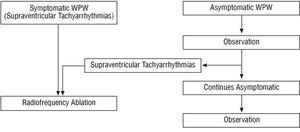
Our proposed general therapeutic strategy is shown in Figure 3. This proposal is in accord with the guidelines for clinical practice.1,2
Fig. 3. Schematic representation of our general therapeutic approach. WPW indicates Wolff-Parkinson-White syndrome-used here in the least restrictive sense in reference to any individual with ventricular preexcitation whether symptomatic or not.
The presence of arrhythmias, whether paroxysmal tachycardias or atrial fibrillation, constitutes one of the indications for catheter ablation because such arrhythmias can cause symptoms and also because they increase the risk of sudden death.
In general, we think it is unacceptable to follow a pharmacological strategy because of the limited information regarding the effectiveness of drugs in protecting against tachyarrhythmias, because of the lack of information about the prevention of sudden death, and because of possible long-term side effects in a chronic disease. In fact, recent multicenter registries of electrophysiological laboratories in Spain suggest that catheter ablation is widespread, as a large number of patients with preexcitation undergo this procedure each year.17,18,47
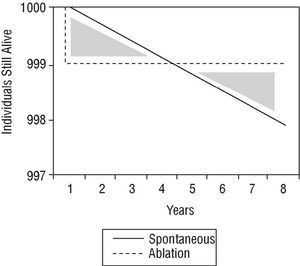
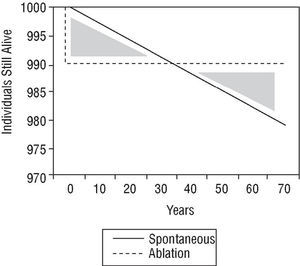
The therapeutic strategy for an individual with asymptomatic preexcitation is subject to controversy. A scientific approach would examine the problem by comparing the risk of sudden death with the risks of ablation procedure, considered as either mortality alone or a composite of mortality and major complications. There are no comparative studies of this nature. However, Figures 4 and 5 show a simple approach to the problem. The ablation procedure is considered to have a success rate of 100% without relapses and any risk is assumed to occur on the day of the procedure. In contrast, the risk of sudden death is considered constant throughout the lifetime of the individual. Two graphs have been plotted. In the first (Figure 4), the only risk from ablation is taken to be mortality and in the second (Figure 5), the risk of mortality is combined with the risk of major complications. In both cases, the only spontaneous risk is sudden death because tachyarrhythmias are considered a treatable event that leaves no sequelae. It can be appreciated that in the first supposition (which only considers risk of mortality), an increase in length of life (end of the area corresponding to both shaded triangles) is reached 8 years after diagnosis. When the increase in length of life without major complications is considered, the same point is reached 70 years after diagnosis. Obviously, this analysis is somewhat artificial and should therefore only be considered as a rough guide.
Fig. 4. Density of incidence of sudden death, plotted over time for an imaginary initial group of 1000 patients. Spontaneous indicates spontaneous clinical course of the patient group; ablation, clinical course of patients treated with catheter ablation who die on the day of the procedure (immediate mortality equivalent to sudden death) but then there are no more deaths, assuming 100% success rate and no recurrences (see text). The shaded triangles represent the difference in mortality in principle favorable to the conservative approach (lives gained). After the curves cross, ablation is favorable (lives lost). Ablation starts to be beneficial from the moment when the triangles have the same area, about 8 years after diagnosis.
Fig. 5. Probability density of incidence of sudden death and major complications (with serious sequelae). The format is the same as Figure 4. Note that ablation starts to be beneficial 70 years after diagnosis with these criteria.
In view of what we have discussed, we think the approach should be guided by the risk ratio of sudden death/risks of the ablation procedure. This ratio does not favor intervention in asymptomatic preexcitation. Nevertheless, we should make clear to patients that if they start to suffer symptoms of tachyarrhythmia, they should consult a doctor as soon as possible because they are probably candidates for ablation. Thus, when these patients undergo regular check-ups, the most important components are the clinical history and the ECG, as examinations such as Holter monitoring or serial echocardiograms are not beneficial. If the initial arrhythmia is preexcited atrial fibrillation, ablation should be considered urgently before discharge from hospital.
At times, we are faced with whether the strategies we have described above should be changed in specific instances of asymptomatic individuals such as those, for example, in "professions of public responsibility" (pilots, drivers, etc) or professional sports players. Firstly, according to the current guidelines, an individual cannot perform his or her activity while the ECG shows preexcitation. If he or she wishes to do so, ablation would have to be performed. Secondly, although the risk of sudden death is almost nonexistent, paroxysmal tachycardia may occur. This may provoke severe symptoms which, in certain work situations, may represent additional risks. This second point should be considered when making such decisions. Nevertheless, we find no reason to propose a generic therapeutic strategy other than those derived from individual considerations based on the criteria presented.
Correspondence: Dr. J. Almendral Garrote.
Servicio de Cardiología (Planta 5).
Hospital General Universitario Gregorio Marañón.
Doctor Esquerdo, 46. 28007 Madrid. España.
E-mail: almendral@medifusion.com