Keywords
INTRODUCTION
Heart failure as a clinical syndrome is the final pathway of many diseases that affect the heart. It begins with changes both in the way the heart operates and in neurohormonal regulation, and causes reductions in functional capacity, retention of liquids, and reduced survival.1 It is a progressive and fatal disease if left to freely develop. Once heart damage is established, a series of compensatory mechanisms are triggered which initially try to maintain cardiac output but, at the same time and in the longer term, they accelerate the deterioration of the heart muscle and cause signs and symptoms of circulatory congestion and low output. In recent years, HF has been become one of the most serious public health problems in developed countries, due to the ongoing increase in its incidence and the personal, social and economic impact that can be expected in the near future. The prevalence of HF in developed countries is around 1%-2% of the adult population2 and increases with age, such that an estimated 6%-10% of patients over 65 years old have HF.3 In Spain, HF is the leading cause of hospitalization in patients over 65 years old.4 It has been found in some series5 that the average age of patients requiring hospital admission for HF is around 75 years, and 30% of these patients suffer from other diseases, such as diabetes or chronic obstructive pulmonary disease (COPD). In addition to high morbidity, HF has bad prognosis with a high number of fatalities, due to sudden death and the progression of HF. Reduced survival is directly correlated to the degree of deterioration in cardiac function. Once HF is diagnosed, 5-year survival is achieved in less than 60% of cases, whereas with serious refractory HF annual survival is less than 25%.6
In recent years, scientific evidence has shown that it is possible to delay the development of the disease via therapeutic approaches based on the pathophysiology of HF.7 Several national and international scientific societies have drawn up consensus guidelines3,8,9 to ensure the greatest uniformity and scientific rigor in the diagnostic and therapeutic management of patients with this syndrome (Table 1).
DEFINITION OF ADVANCED OR REFRACTORY HEART FAILURE
Advanced or refractory HF can be defined as the persistence of symptoms that limit daily life (functional class III or IV of the New York Heart Association [NYHA]) despite optimal previous treatment with drugs of proven efficacy for the condition, i.e. ACE inhibitors, angiotensin II receptor antagonists (ARA-II), diuretics, digoxin, and beta-blockers.10 According to the latest classification proposed by the ACC/AHA,3 this corresponds to stage D heart failure. This refers to patients with advanced structural heart disease and severe signs of HF at rest who are candidates-in the absence of contraindications-for other specialized interventions, such as heart transplantation (HT), ventricular remodeling, implantation of mechanical assistance devices or the administration of intravenous inotropic drugs. Terminal HF is the last step in advanced HF, where there is a very poor response to all forms of treatment (by definition, HT is no longer indicated), with serious deterioration of quality of life-both physical and emotional-frequent hospitalization and life expectancy less than 6 months.
Evaluation of the Patient With Refractory Heart Failure
The evaluation of patients with HF has the following aims: a) searching for potentially treatable and reversible factors; b) correct characterization of the symptoms; and c) defining the hemodynamic profile with a view to designing treatment.
Search for Potentially Treatable Factors
Anemia, pulmonary embolism and infections are usual causes of decompensation. Common viral infections frequently cause persistence of symptoms of decompensated HF for several weeks after the resolution of the viral picture. Thyroid dysfunction is a cause of decompensation and should be ruled out, especially in patients undergoing amiodarone treatment. Excessive alcohol consumption can cause cardiomyopathy and/or aggravate the HF situation, although alcohol intake in small quantities has been associated with a lower incidence of HF in some epidemiological studies.11 Atrial fibrillation is involved in 25%-50% of patients with refractory HF. Rapid ventricular response can be both a cause and a consequence of decompensated HF, so both aspects should be taken into account. Once the clinical situation has been stabilized, it remains a matter of debate whether restoring sinus rhythm is preferable to maintaining strict control over heart rate. Although digoxin is effective in controlling the heart rate at rest, the use of beta-blockers or amiodarone is usually required during exercise. Obesity increases the risk of developing HF12,13 and decompensation of previous HF. Furthermore, there is a form of cardiomyopathy associated with obesity that can be reversed after sufficient weight has been lost. Hence the importance of the patient avoiding excess weight.10 The presence of underlying ischemic heart disease is a potentially treatable etiological factor. Coronary disease affects 50%-70% of patients with advanced HF,10 although the search for reversible ischemia is not always easy. According to data from controlled studies and/or registries, revascularization is indicated when the left ventricular ejection fraction is between 35% and 50% or lower than 35% but with symptoms of ischemia.14 Although non-invasive studies have been frequently done to verify whether there are ischemic regions which can be revascularized, no clear evidence from controlled studies exists supporting the usefulness of intervention in the absence of angina.15,16
Ventricular surgery techniques have aroused great interest. In patients with non-ischemic dilated cardiomyopathy, partial left ventriculectomy (Batista procedure), together with surgery of the mitral valve, was a very popular technique some years ago. It had a sound theoretical basis, i.e. the premise that structural remodeling of the heart would make it possible to reduce stress on the ventricular wall. However, this technique is currently in disuse due to the bad clinical results obtained, especially in studies carried at the Cleveland Clinic.17 In fact, in the latest AHA/ACC guidelines,3 the Batista procedure features as a type III indication. However, in patients with ischemic heart disease and dyskinetic regions, aneurysmectomy, and endoventricular circular patch plasty (Dor procedure) seems to be a very promising technique, since it stops or prevents ventricular remodeling and can delay or prevent HT. Although this technique has been used since 1984,18 its use is still limited to particular centers. Nevertheless, many series exist,19,20 some including more than 1000 patients,18 with good results.
Characterization of the Symptoms
Correct identification of the patient's symptoms is important to identify those causing the main restrictions in daily life. When assessing functional limitation, the NYHA classification sometimes lacks precision (e.g. it can be difficult to distinguish between class II and III), which means that it can be useful to regularly review changes in the capacity of the patient to carry out normal activities, such as getting dressed, having a walk around the block, going up the stairs, pushing the shopping cart, etc. Symptoms of advanced or refractory HF are the result of 2 pathophysiological mechanisms, congestion and low output, and either can predominate in a given patient. In relation to lung or systemic congestion, HF symptoms are due to elevated left or right filling pressures, respectively. The former causes exertional dyspnea, orthopnea, cough with decubitus, or dyspnea with minimal effort or at rest. The latter causes edema, ascites, anorexia, easy satiety, undernutrition, and discomfort when the patient tries to stoop or bend the spine. Symptoms attributable to low heart output are sometimes less specific, such as asthenia, lack of energy, easy fatigue and depression, and/or irritability related to the impossibility of carrying out normal activity. As a consequence of a low cerebral perfusion, there can also be changes in nocturnal sleep patterns, drowsiness, or concentration difficulties.
Definition of the Hemodynamic Profile
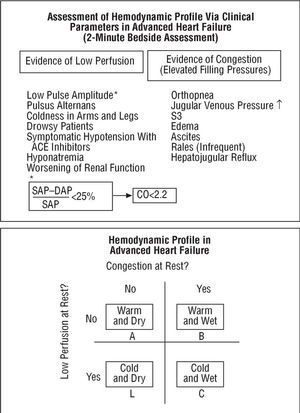
The therapeutic approach to HF differs depending on whether symptoms of congestion or low output predominate.10,21 Precisely establishing the severity of lung or systemic congestion and cardiac output requires direct determination of filling pressures and heart output via right heart catheterization.22,23 However, another practical, simple and fast method has been proposed to do this non-invasively using the 2-minute bedside assessment technique. This examination makes it possible to classify hemodynamic profiles into 4 categories, taking into account the presence or absence of congestion and low output, as defined by clinical parameters (Figure 1). In most cases, this clinical measurement has prognostic value24 and seems to be a sufficient guide to treatment,10 while maintaining as general objectives: normal venous pressure, the disappearance of edema and orthopnea, systolic pressure >80 mm Hg, stable renal function, and the capacity to walk on a level surface without fatigue. Hemodynamic study, that is, right heart catheterization, is reserved for special cases, the measurement of pulmonary hypertension or when another disease, e.g. sepsis, complicates the development of HF and hinders accurate characterization of the situation.
Fig. 1. Most patients can be classified, by bedside assessment, into four types of hemodynamic profiles that are very helpful in guiding treatment and establishing prognosis. Adapted from Nohria et al.10 CO indicates cardiac output; DAP, diastolic arterial pressure; SAP, systolic arterial pressure.
PHARMACOLOGICAL TREATMENT OF ADVANCED HEART FAILURE
The primary aim of HF therapy is to relieve symptoms, followed by preventing disease progression and prolonging survival. As the disease progresses, the probability of successfully achieving these objectives gradually diminishes until, in the most advanced stages, it is only possible to achieve symptomatic control. It is assumed that patients with refractory HF have had previous treatment with diuretics, ACE inhibitors, digoxin, spironolactone, and beta-blockers.
In the past, HF was interpreted as a change to low cardiac output and treatment aimed at attempting to improve this with inotropic drugs. However, it was shown that this treatment was deleterious, since it caused an increase in mortality.25-27 Subsequently, treatment was based on attempting to reduce filling pressures, whether accompanied by tissue hypoperfusion or not,22 and this improved heart function. At present, the benefits of reducing congestion are indisputable and go beyond symptomatic relief. Reducing filling pressures lessens the severity of mitral regurgitation and consumption of myocardial oxygen. It also improves myocardial perfusion, thus helping to reduce ischemia in patients in whom HF is due to coronary disease. Furthermore, reducing filling pressures lowers neurohormonal activation.28 In the end-stages of HF, the most frequent obstacle to management is the so-called cardiorenal syndrome. As diuresis relieves congestion some patients undergo deterioration in renal function, especially those who have had previous renal dysfunction. This is particularly frequent in cases of chronic volume overload, right ventricular failure and baseline requirements of high doses of diuretics. This was initially attributed to a situation of prerenal kidney failure, but this explanation is currently inadequate, since it is known that at times it takes place with filling pressures that exceed optimal levels of cardiac output. Many factors have been proposed, such as the interaction of vasodilator and vasoconstrictor hormones and not well understood triggering mechanisms. This often makes it difficult to decide whether to treat renal function or improve symptoms. The symptoms can improve at the expense of worsening renal function but, on the other hand, the progressive increase in urea and creatinine levels is correlated with a greater probability of hospitalization and death. There is no simple solution to this problem and it requires better understanding of the mechanism that produces this syndrome.
TREATMENT ACCORDING TO DIFFERENT HEMODYNAMIC PROFILES
Patients with HF can be rapidly classified into one of 4 hemodynamic profiles by use of medical records and physical exploration (Figure 2):
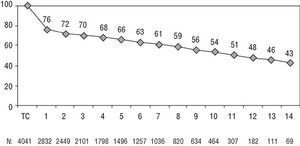
Fig. 2. Registro Español de Trasplante Cardíaco (Spanish Cardiac Transplantation Registry). Actuarial survival. Taken from Almenar Bonet.45
- A: warm and dry.
- B: warm and wet.
- C: cold and wet.
- L: cold and dry.
Profile A: Warm and Dry
These are the patients without evidence of elevated filling pressures or low output. Treatment is directed at maintaining an adequate filling volume and preventing disease progression. In patients with resting symptoms who present this profile it is worth asking whether the symptoms are really due to HF.
Profile B: Warm and Wet
These are the patients who present congestion without low output. The first aim is to increase diuretic treatment. They can frequently be treated with oral diuretics on an outpatient basis, especially if the decompensation is of recent onset. If there is no improvement, hospitalization is required for treatment with intravenous loop diuretics (bolus or continuous infusion) and at times it is necessary to resort to powerful diuretics such as metolazone; the administration of this drug requires close monitoring of potassium levels.29 Congestion can be relieved with vasodilators, such as intravenous nitroglycerin or nesiritide.
Nesiritide is a recombinant form of the endogenous type B natriuretic peptide that has recently been approved by the Food and Drug Administration (FDA) for the treatment of decompensated HF. It seems to be effective in rapidly reducing congestion symptoms.30,31 It binds to receptors in the vessels, kidney, adrenal glands and brain, and it reverses resistance to endogenous BNP present in patients with advanced HF. It acts as an arterial and venous vasodilator, increases natriuresis and suppresses the activation of the renin-angiotensin-aldosterone and adrenergic systems. It has been found that it can strengthen the effect of diuretics in some patients. The main risk is hypotension, which is a little more prolonged than with nitroglycerin, since the half-life of nesiritide is around 18 h.
Although diuretic treatment is essential to relieve congestion, it has adverse effects that limit its use, such as hypotension, electrolytic alterations (hypomagnesemia, hypokalemia, hyponatremia), and worsening of renal function. Thus, other drugs could be useful, such as antidiuretic hormone arginine vasopressin (AVP) antagonists, that are currently under study. The AVP hormone, which has a powerful vasoconstricting and modulator action on the transportation of free water in the kidney, plays an important role in the normal regulation of cardiovascular physiology. The values of AVP are elevated in HF and their increase is associated with disease progression. At least two types of AVP receptors are known: V1A receptors, which cause vasoconstriction and mitogenesis in vascular smooth muscle cells, and V2 receptors, that reduce the excretion of free water and modify the reabsorption of sodium and urea in the kidney. A third group of receptors, V3 (V1B), participates in the regulation of the hypothalamic-pituitary-suprarenal axis, but has a limited role in HF. Tolvaptan is a selective AVP V2-receptor antagonist32 and, due to its capacity to eliminate free water, it is believed that it can be useful in managing congestion in HF. The ACTIV in CHF study (Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure), a randomized clinical trial of tolvaptan versus placebo, has shown that that drug, when added to standard treatment in patients with acute decompensated HF, seems to be useful in reducing congestion without modifying heart rate, blood pressure, potassium concentration or renal function.33 The long-term usefulness of this drug will be understood bette r with the data from the EVEREST study (Efficacy of vasopressin antagonism in heart failure: outcome study with tolvaptan), currently in phase III, in which the efficacy of tolvaptan versus placebo is compared in adults with decompensated HF. Some authors have expressed their concern over the theoretical possibility of this drug--given its action as a selective V2-receptor antagonist--increasing the interaction of AVP on non-blocked V1A receptors, thus causing vasoconstriction, afterload increase and worsening of ventricular function. In this regard, it is worth asking whether a dual V1A- and V2-receptor antagonist, such as conivaptan, could improve the results.34 However, more studies are still needed to better understand the usefulness of these drugs.
Inotropic treatment is not necessary in these profile B patients. If they are receiving chronic outpatient treatment with beta-blockers, there is no reason to withdraw it. However, if decompensation coincides with an increase in beta-blocker dosage, it is advisable to reduce the dose to that prior to destabilization.
Profile C: Cold and Wet
These are the patients who present congestion and low output. "Heating then drying" these cold and wet patients is required, that is, to improve perfusion then to relieve congestion. Beta-blockers and ACE inhibitors should be suspended until the patient is stabilized. It is necessary to optimize cardiac output with inotropic treatment, and when this improves, it is time to "dry" the patient by using diuretics. There is debate concerning the relative benefit of the inotropic and vasodilator effects of several drugs, such as dopamine at low doses, dobutamine, or milrinone. Nesiritide can also be useful in this group of patients. In a random study of 261 patients with acute decompensated HF, nesiritide was compared to dobutamine and, in the short-term, the former was more effective than the latter in reducing mortality and costs.35 Levosimendan is another recently introduced drug that sensitizes C protein to calcium depending on its concentration, which means that it does not affect diastole, having inotropic and vasodilator effects that improve cardiac output without increasing myocardial oxygen consumption. In a random study of 203 patients with HF and severe low output, levosimendan proved more effective than dobutamine in improving hemodynamic parameters.36
When hypotension impedes the withdrawal of intravenous inotropic drugs, ACE inhibitors need to be stopped. In some patients, hydralazine alone, or in combination with nitrates, can be of use when withdrawing intravenous inotropics.
Although the benefits of inotropic treatment do not always justify the risk of arrhythmias (sometimes fatal), it is at times the only treatment that makes it possible to keep patients with severe hemodynamic instability alive. At the same time, when the hemodynamic situation is initially a little confusing, an intravenous infusion can provide hemodynamic stability while obtaining a more accurate hemodynamic profile. Inotropic treatment is also useful in patients with chronic decompensation when there is a worsening of renal function, with elevated urea and creatinine levels, and where sufficient diuresis is not obtained despite using high-dose loop diuretics combined with thiazides. In general, the use of inotropics in HF should be regarded as a temporary therapy, a type of "bridge" to obtain diuresis, until transitory concomitant diseases such as pneumonia are resolved or until transplantation. With the aim of resolving congestion without worsening cardiac output, new strategies have recently been described which cast doubt on concepts that so far have been considered as dogma in the management of advanced HF. These include the use of hypertonic saline in combination with high-dose diuretics and a diet with a moderate reduction of sodium (less restrictive than the amount of sodium classically advised in HF) to obtain effective diuresis.37,38 Although the studies included a low number of patients, the strategy seems to be beneficial, without precisely knowing which of the components (the hypertonic saline or the moderately low sodium diet) have the greatest influence on the results.
Profile L: Cold and Dry
These are the patients with low output and without congestion. Many of the patients classified with this profile have congestion, although it is clinically negligible. Patients with low output and without clinical evidence of elevated filling pressures can be, surprisingly enough, clinically stable for a long time and generally do not present urgent symptomatology. In cases in which the filling pressures are below normal values, volume therapy should be started, and when there is excessive vasodilatation, vasodilator treatment should be withdrawn or reduced. If neither of these two conditions is present, the management of these patients is much more difficult. Intravenous inotropics can lead to dependency and tachyphylaxis. A gradual introduction of beta-blockers, if well-tolerated, can be beneficial,39 especially if the heart rate is high. However, no clinical trials have been done with this group of patients.
MANAGEMENT OF TERMINAL HEART FAILURE PATIENTS
A terminal situation is understood as an incurable disease (whether due to the lack of response to treatment, or because there is no curative treatment), that threatens the patient's life in the short-term, generally in less than 6 months, and produces progressive symptoms that seriously affect the functional capacity and emotional state of the patient and, by implication, the family's. Although many aspects related to the management of terminal situations are common to any progressive chronic disease in its final phase, in HF there are particular aspects worthy of comment.
Entry into the terminal phase of HF, as in other chronic diseases, is poorly-defined, thus hindering decisions such as when to begin purely palliative care. The prognostic factors that can help predict the survival time of the patient with advanced HF are:
- Clinical: ischemic heart disease as etiology, sustained arterial hypotension, NYHA classes III and IV, absence of triggering factors eligible for treatment, and the presence of other concomitant diseases that limit survival.
- Hemodynamic: left ventricular ejection fraction less than 20%, low cardiac output, arterial hypotension, sinusal tachycardia, and kidney failure.
- Biochemical: high values of norepinephrine and natriuretic peptides, and hyponatremia.
- Electrophysiological: presence of potentially serious arrhythmias.
The factors which seem to be better predictors of evolution are ejection fraction, functional class, hyponatremia and the type of heart disease. In practice, patients with advanced decompensated HF can often be stabilized to avoid new acute episodes and they can sometimes be stable for periods of unpredictable duration, although these can be prolonged. Given continuous aggravation from the disease, it must be verified that there is good dietary and pharmacological compliance, treatment adjusted as necessary, the need for oxygen therapy at home or at the hospital assessed, and the family and patient kept properly informed about the real situation.
The usual profile is a patient in NYHA functional class IV, with bad response to conventional treatment, with no indication for HT, and where he/she is experiencing serious deterioration in the quality of life, great discomfort and repeated hospitalizations in the previous months. Dyspnea (80%) and pain (40%) are the most frequent symptoms in these patients, along with restlessness, anorexia and digestive problems. Several studies have shown that discomfort and stress in these patients can become even worse than in patients with cancer.
It is advisable to provide the patient and family with health education on what can be expected regarding the disease and possible final treatment options. This should be done before the patient is too sick to participate in decision-making. During such discussions on treatment preferences, the possible circumstances (which require different management) that frequently occur in the context of terminal HF should be taken into account, such as reversible aggravation of the HF situation, cardiac arrest, a serious sudden event (e.g. stroke) or the worsening of concomitant non-cardiac disease (e.g. kidney failure). A suitable review of these possible diseases with the family will help them understand the differences (and, thus, the degree of relevance) between the possible treatments. Thus, for example, rapid intervention would be justified to correct a reversible situation, but maintaining life support to continue life indefinitely without reasonable expectation of returning to good functional capacity would probably not be justified.
Regarding cardiac arrest, some studies suggest that, in contrast to other chronic diseases, most patients hospitalized for advanced HF prefer resuscitation maneuvers to be carried out. In a study40 of 936 patients hospitalized for advanced HF, where their preferences were analyzed regarding cardiac arrest, as well as how firm these decisions were, just 23% expressed their desire not to be resuscitated in case this event happened. The circumstances associated with this decision were old age, the perception of poor prognosis by the patient, the worst functional class and prolonged hospital stay. Furthermore, when 600 of them were asked the same question again after 2 months, it was found that 19% had changed their preferences, that is, 14% of the patients who had initially desired resuscitation and 40% of those who did not. The desire of the majority to be resuscitated contrasts with the preferences of terminal patients with other chronic diseases,41 perhaps because HF patients frequently have had the opportunity to experience long periods of clinical stability when they have enjoyed good quality of life, even after having been hospitalized in intensive care units due to severe worsening. In the previously cited study, the decisions made by the patient could rarely be changed after discussion with the medical team.
However, when the limitations caused by HF on its own or in combination with other concomitant diseases become intolerable for the patient, resuscitation is frequently not desired. In this situation it is important to know the desires of the patient in order to direct clinical management, from non-resuscitation to, for example, non-hospitalization. In the event that the patient has an automatic implantable defibrillator, its deactivation would probably be indicated to provide the patient with the possibility of sudden death instead of death as the result of the progression of congestive HF. In any case, it is strongly advisable that the management of the patient, both in hospital services at home and in conventional hospitalization, is undertaken or coordinated by the same team so that both the patient and family perceive a uniformity of criteria in the healthcare plan.
Many of these patients with symptoms such as dyspnea, pain or restlessness, die unnecessarily in acute hospitals, where the aims are more curative than palliative, such as those designed to alleviate symptoms and improve quality of life in this last stage of the disease.42
Home-based hospital services are of great help to patients with terminal HF. Originally, these were specifically designed for the relief of terminal cancer patients, but currently their functions have been expanded to other types of diseases that require relief from symptoms other than pain, such as dyspnea in the case of the HF. Patients with terminal HF frequently present congestion-related dyspnea. To relieve the symptoms, intravenous diuretics or even intravenous infusion of positive inotropic agents in some cases can be more effective than the mere use of powerful analgesics. These patients very frequently complain of pain, without any clear location, as one of the most worrying problems. During the final days, management often requires the use of tranquillizers and narcotics. Suitable treatment for terminal HF should be maintained during this final phase and, if needed, palliative treatment as follows:
- Morphine at a dose of 3-5 mg/4 h given subcutaneously or immediate-release morphine via oral at a dose of 10-20 mg/4 h. The dose should be increased until the desired response is obtained then changed to controlled-release morphine. Morphine is a very useful vasodilator and tranquilizer, and helps reduce the work of breathing. It is also very effective for pain secondary to myocardial ischemia.
- Diazepam, beginning with 5-10 mg/8-12 h, depending on patient response. Diazepam lowers anxiety which on the other hand perpetuates dyspnea.
- Oxygen therapy, which is frequently prescribed to cope with dyspnea, since its effect is basically psychological.
HEART TRANSPLANTATION
Progress in the medical treatment of HF, as well as in transplantation, has shown that HT especially benefits the population of patients with a high risk of death from advanced terminal HF.43,44 On the other hand, heart transplantation is limited by the insufficient number of donors and contraindications to this procedure. At present, given the progress in many aspects of HT, it is uncommon to talk in terms of absolute or relative contraindications, but rather of conditions that increase the risk of post-HT morbidity and mortality. These include old age, concomitant diseases (e.g. diabetes with visceral disorder, kidney or liver failure, COPD, background of neoplasms or diseases with bad short-term prognosis) and psychosocial conditions that involve poor commitment to treatment. These 2 limitations make HT "epidemiologically" irrelevant, especially when we take into account that the number of HT carried out in Spain is around 300/year45 (the number of donors per million inhabitants is still the highest in the world) in contrast to the growing incidence of HF which is estimated in thousands of cases per year.
According to the most recent guidelines of the ACC/AHA3 (Table 2), the absolute indications for HT in HF patients include the following: a) refractory cardiogenic shock; b) dependency on intravenous inotropic drugs, and c) persistent NYHA class IV symptoms with oxygen consumption less than 10 mL/kg/min. The expected benefit for this group of patients is 50% survival at 1 year without HT, versus around 80% survival at 1 year and 50% survival at 10 years with HT.45,46 Most patients undergoing outpatient oral treatment have a relative indication for HT, i.e. this is indicated although the degree of benefit is not as high as in the previous group. This group includes patients who present functional limitation due to oxygen consumption between 11 mL/kg/min and 14 mL/kg/min and those in whom, once their correct adhesion to treatment has been verified, it is difficult to obtain a balance between the absence of congestion and low output. In the absence of other indications, a low ejection fraction or a history of having been in NYHA functional class IV is no longer taken as an indication for HT. The benefit of HT in patients with stable HF is not as clear, in the light of the latest improvements in the medical treatment of HF. Some authors have even proposed that this aspect should be better studied through a randomized clinical trial.47
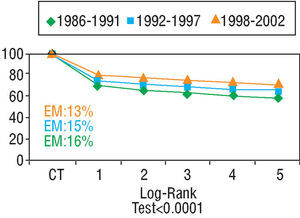
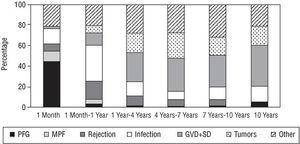
Heart transplantation has dramatically improved in the last 20 years, basically as a result of improvements in surgical techniques, breakthroughs in immunosuppressive treatment and in the management of infection. According to data from the Registry of the ISHLT46 and the Registro Español de Trasplante Cardíaco45 (RETC) (Spanish Registry of Heart Transplantation), 1-year survival is around 80% and at 10 years around 50%-60% (Figures 2 and 3). Morbidity and mortality after HT is particularly related to surgery, rejection (in its different acute and chronic forms), and the toxicity of immunosuppression, especially infections and neoplasms. The most frequent causes of death in the first year are primary failure of the graft and multiorgan failure, followed by rejection and infection. In the long term, the most frequent individual causes of death are graft vascular disease and neoplasms (Figure 4).
Fig. 3. Registro Español de Trasplante Cardíaco (Spanish Cardiac Transplantation Registry). Survival by period. EM indicates early mortality. Taken from Almenar Bonet.45
Fig. 4. Registro Español de Trasplante Cardíaco (Spanish Cardiac Transplantation Registry). Causes of mortality by period. PFG indicates primary failure of the graft; MOF, multiorgan failure; GVD, graft vascular disease; SD, sudden death. Taken from Almenar Bonet.45
Basal immunosuppression usually involves three-fold therapy, consisting of a calcineurin inhibitor (CNI) such as cyclosporin A (CsA) or tacrolimus, an antiproliferative such as mycophenolate mofetil (MMF) or azathioprine, and steroids. Induction therapy can be useful in the immediate postoperative period, especially because it makes it possible to delay treatment with CNI to prevent the nephrotoxicity associated with these drugs. The antilymphocyte antibodies used in the past as induction therapy, whether anti-CD3 monoclonal antibodies (OKT3) or polyclonal antibodies (e.g. ATG), are currently being replaced with new monoclonal antibodies directed against interleukin-2 receptors, since they seem to be less toxic and have similar efficacy.48,49 High doses of corticosteroids, either intravenous (250 mg to 1 g/day, 3 doses) or orally, in descending doses, are used to treat acute rejection episodes. If rejection cannot be controlled, antilymphocyte antibodies (OKT3, ATG) and modification of the basal immunosuppression are used. Plasmapheresis sessions are useful where humoral rejection occurs.
The CNI specifically act on the immune system without affecting other cells which proliferate rapidly. Their main mechanism of action is done through binding to specific proteins, and thus forming complexes that block the action of calcineurin, which is vital to the activation of T cells. In this way, CNI block the transduction signal that causes the activation of the T and B cells. Cyclosporin A was the first CNI and the most widely used in HT. In some countries such as Spain, it is currently the only CNI with approved indications for primary immunosuppression. The original oil-based formulation of CsA had rather unpredictable pharmacokinetics, but was improved at the beginning of the 1990s with the new microemulsion formulation called Neoral®. Some studies showed that, compared to the standard formulation, Neoral® reduced the number of rejection episodes and the incidence of infections.50 It is currently the CsA formulation of choice. Several prospective studies have confirmed that standard formulation CsA and tacrolimus, in regimens associated with azathioprine and steroids, have similar efficacy in the prevention of acute rejection and survival at 5-year follow-up.51 The efficacy of Neoral® CsA, in contrast to tacrolimus in regimens associated with MMF and steroids, will be better understood when the results of the current multicenter European PANEUHTX (Paneuropean Heart Transplantation) study are reported. All these studies measured CsA exposure with the standard procedure by CsA (C0) trough values. However, it is now known that the concentration of CsA 2 h after administering the dose (C2) seems to be a better predictor of CsA exposure, although it remains to be demonstrated whether the short- or long-term efficacy of CsA in the prevention of rejection or improvement in survival is better when controlled via C2. Although some preliminary works on control with C2 in the long-term HT52,53 are available, there is no consensus on the level of C2 to be reached. The MOTOWN study (Monitoring of Sandimmune Neoral Two hOurs absorption With simulect in heart transplantatioN), currently in progress, will provide information in this regard. A drawback to controlling with C2 is the need for a change in hospital logistics, since it is essential to do the test at 2 h (±15 min) after the dose, which is sometimes difficult to fulfill in daily clinical practice. Given that CsA and tacrolimus show similar efficacy, the determining factors when selecting one or the other are their side effects. The most important adverse effect is nephrotoxicity which happens with similar frequency with the 2 CNI. According to the literature, there might be 2 cardiovascular risk factors, hypertension, and hyperlipidemia, that have a lower incidence with tacrolimus; on the other hand, there is a lower incidence of diabetes with CsA. This can help decide which of the CNI would be more suitable according to the profile of each patient.
Azathioprine, a purine biosynthesis inhibitor, was initially the antiproliferative of choice, and its greatest side effect was hemotoxicity. Mycophenolate mofetil, which has a similar action (inhibitor of de novo synthesis of purines) but is more selective for lymphocytes, demonstrated a reduction in mortality at 1 year and in the severity of rejections in a double-blind clinical trial54 versus azathioprine when used in association with CsA and prednisone. This benefit in survival was also found at 3 years based on the data from registries.55 Thus, it is currently the drug of choice as an antiproliferative.
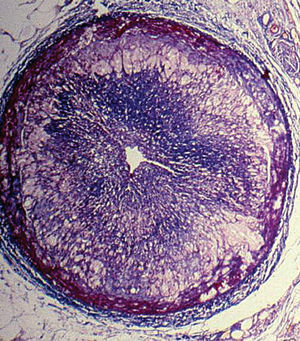
Graft vascular disease (GVD) or chronic rejection is one of the most frequent causes of death in the long term. It manifests as a diffuse, concentric and gradual obliteration of the graft vessels (Figure 5) with multifactorial etiology in which immunological and non-immunological mechanisms are involved. Conventional therapies of coronary revascularization (surgical and/or percutaneous) are only palliative and classical immunosuppression has proven to be ineffective, both in the prevention and treatment of this complication. The new antiproliferative drugs, mTOR inhibitors (mammalian Target Of Rapamycin), everolimus, and sirolimus are promising regarding the prevention of GVD. In an international clinical trial it was demonstrated that everolimus associated with CsA and steroids reduces the progression of GVD compared to azathioprine, in addition to reducing the rate of acute rejection.56 However, in this study, everolimus was associated with a worsening in renal function attributable to an increase in CsA exposure. The use of low-doses of CsA would possibly attenuate this side effect, although more studies are needed to find out what dose and optimal level of CsA should be employed.
Fig. 5. Graft vascular disease. Micrograph of an epicardial coronary artery where we see an occlusion of the lumen secondary to intimal proliferation (Masson trichrome x2.5).
INOTROPIC TREATMENT
The increase in the contractile strength of the heart is an important mechanism to improve cardiac output in advanced HF. However, experience with oral inotropics has been negative since, although hemodynamic parameters improved in the short-term, clinical data showed that prolonged oral use of positive inotropics increased mortality.26,27
The reduction in cardiac output at rest reduces blood flow to the vital organs, especially to the kidneys. In this circumstances, only positive inotropic drugs are capable of increasing systolic volume, both the classical ones (dobutamine, dopamine, milrinone, adrenaline, norepinephrine) and the recently introduced calcium sensitizers, such as levosimendan,36 in combination or not with vasodilators (nitroglycerin, nitroprusside). The decision to use inotropic treatment and the selection of the inotropic agent should reflect the therapy objectives for each particular patient:
- Critical support until definitive treatment (cardiogenic shock).
- Support until resolution of other conditions that have caused decompensations in the patient; whether cardiac (e.g. recovery after a sustained ventricular tachycardia or heart attack) or non-cardiac (e.g. pneumonia or gastrointestinal hemorrhage).
- Resolution of a congestion with worsening of renal function.
- As a bridge therapy to HT and/or circulatory mechanical assistance.
- Bridge to the end of life.
Patients with refractory HF tend to be hospitalized due to clinical deterioration which usually manifests as congestion and/or low output. Given low output, and once hypovolemia is ruled out, i.e. there is a "cold and wet" hemodynamic profile, inotropic treatment is essential to improve contractility, facilitate diuresis and clinically stabilize the patient.57
Patients in whom the attempt to withdraw inotropic treatment fails can require the placement of a permanent central catheter to allow the prolonged use of inotropics (usually dobutamine or milrinone). This treatment is frequent in patients awaiting HT, but it can also be contemplated in non-candidates to HT, those terminally ill and those dependent on inotropics who, therefore, could not otherwise be discharged from hospital. The decision to administer inotropic treatment at home should not be offered until all the other possible alternatives have been tried. Infusion of inotropics at home involves an increase in the family's workload and in the consumption of health resources and, finally--which is more worrying--, this increases the risk of death due to probable arrhythmic causes. However, continuous inotropic support can lead to symptomatic improvement and avoids hospitalization. This is one of the aims of a global plan to make it possible for the patient to die in a state of well-being and at home.57
Such use of inotropics at home is totally different to the intermittent infusions of intravenous inotropics in patients in whom inotropic treatment had been successfully withdrawn previously, since the results of studies do not support this therapy. The OPTIMIZES-CHF58 study (Outcomes of a prospective trial of intravenous milrinone for exacerbations of chronic heart failure) is a randomized trial where intravenous milrinone was compared to placebo in 951 patients hospitalized for decompensated HF. Inotropic treatment was indicated for these patients, but was not essential.59 In fact, patients with renal failure, persistent arterial hypotension (systolic blood pressure less than 80 mm Hg), and atrial fibrillation with heart rate higher than 100 beats/min were excluded. The main aim was to determine whether milrinone could reduce the number of days in hospital due to cardiovascular causes 60 days after admission. There were no differences in hospital time, and the group with milrinone presented a greater incidence of arrhythmias and mortality, both intrahospital and in the following 60 days. Thus, intermittent infusions of intravenous inotropics in stable patients do not seem to be an effective therapy.60
A cardiogenic shock requires immediate treatment with inotropics until the cause of the shock is determined and definitive treatment established. Acute stimulation of contractility increases cardiac output and blood pressure. The initial agent of choice is normally dopamine at medium to high doses (5-25 µg/kg/min), followed by dobutamine and milrinone, since the latter, due to their peripheral vasodilator effect, tend to be less useful. In severe vasodilatation situations, alpha- and beta-agonists such as adrenaline and norepinephrine are useful if vasodilatation persists. However, it should be born in mind that inotropic treatment can trigger atrial and ventricular arrhythmias and that the increase in contractility and heart rate can increase oxygen demand, although at times this is compensated for by the reduction in ventricular volumes and wall stress. The use of adrenaline and norepinephrine is restricted to situations of imminent risk of death, due to high risk of arrhythmias, myocardial ischemia and compromised peripheral circulation. The following strategy describes the establishment of circulatory mechanical support in suitable candidates.
VENTRICULAR ASSISTANCE
Mechanical assistance devices (MAD) were initially used as temporary support for patients with severe HF in a critical situation. This included patients on the HT list, in a state of cardiogenic shock and awaiting an available donor ("bridge to transplantation"), or those in situations of serious but reversible cardiac dysfunction, such as acute myocarditis or postsurgery cardiac dysfunction ("bridge to recovery"). Subsequently, and after having achieved long survival periods with these devices given the previous indications, a second aim of MAD was considered to be definitive support for patients with terminal HF. Definitive support versus HT would have the following advantages: eliminate the limitations imposed by the insufficient number of donors, eliminate the need for chronic immunosuppression, and make it possible to decide the moment of implantation depending on the clinical situation of the patient.
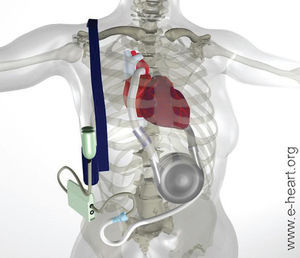
The REMATCH study (The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure)6 was the key clinical trial in assessing the usefulness of mechanical assistance devices as treatment for refractory HF. This was a random, prospective, multicenter study done in the United States to determine whether left ventricular assistance (LVA) with the Thoratec HeartMate device (Thoratec, Pleasanton, CA) was better than optimal medical treatment in patients with advanced terminal HF and ineligible for HT due to the presence of contraindications (Figure 6). The main aim of the study, which included 129 patients, was global survival and 2-year follow-up. Inclusion criteria were: New York Heart Association class IV HF for at least 90 days despite treatment with ACE inhibitors, diuretics and digoxin. All the objective data referring to the selection criteria of these patients indicate that this clinical trial included patients with a far more advanced grade of HF than any other clinical trial of terminal HF, both medical and surgical, carried out to date (75% mortality at 1 year in the control group). The group with the left ventricular assist device (LVAD) showed a statistically significant benefit regarding 1-year survival (52% vs 25%; P=.002) and 2-year survival (23% vs 8%; P=.09). The use of LVAD involved a 48% reduction in risk of death compared to medical treatment, with an average survival of 408 days in the LVAD group in contrast to 150 days in the medically treated group. The objective measurements of physical and emotional parameters were also significantly higher in patients fitted with LVAD, which shows that this device had the capacity to improve both the duration and quality of life. Quality of life was better in the patients fitted with LVAD according to some indexes, but this was not the case with others, as those assessed by the MLWHFQ (Minnesota Living With Heart Failure Questionnaire).
Fig. 6. Left ventricular assistance device used in the REMATCH study. The inlet cannula is inserted in the apex of the left ventricle and the outlet via anastomosis in the ascending aorta. The blood returns from the lungs to the left side of the heart, leaves by the cannula and, via an entry valve, passes to the ventricular assist device which is set in place on the abdominal wall or in the peritoneal cavity. The blood is then actively pumped through an output valve to the ascending aorta. A percutaneous cable connects to the battery and the electronic controls.
However, there are several less positive aspects to this trial that should be mentioned.61,62 Global survival, even with LVAD, was low at 2 years (23%). Patients with LVAD suffered 2.35 times more adverse effects than patients with medical treatment which were especially related to infectious, hemorrhagic, and neurological complications and/or malfunctioning of the device (35% failure at 2 years). A subanalysis demonstrated that the benefit in survival only affected the patients who, when randomizing, were in NYHA functional class IV and receiving inotropic treatment. In these patients, mortality after 1 year with LVAD decreased from 76% to 51%. Those patients who were hemodynamically stable with oral treatment showed no benefit in survival.
A study initiated in March 2000 on the feasibility of LVAD as definitive treatment is currently under way. It is known as INTrEPID (Investigation of Non-Transplant Eligible Patients who are Inotrope Dependent), and the LVAD used is WorldHeart's Novacoris. The study includes patients with terminal HF ineligible for HT and who are under treatment with intravenous inotropics. This study is investigating the efficacy of the Novacor LVAD as long-term support for these patients, while comparing the survival rate and quality of life to that of patients undergoing pharmacological treatment. Both devices (Thoratec-HeartMate and Novacor) are implanted next to the heart to assist the left ventricle and use an external battery to power the LVAD. The most significant difference between them is that the patients fitted with the HeartMate do not need anticoagulation therapy.
Despite these observations, it is indisputable that the REMATCH study provided very valuable information. Even with first-generation devices, the patients fitted with an LVAD who had an average age of 68 years, and all the problems related with the device, improved compared to patients receiving medical treatment only, at least in the short- to medium term. Without doubt, given a better selection of patients and advances in LVAD technology, global surv ival can be improved in this cohort of patients with such a high risk of death. In November 2002, the FDA approved the HeartMate device as definitive treatment for patients ineligible for HT. Consensus documents have been prepared with the aim of improving the implementation of mechanical assist devices as definitive therapy. These documents describe the requirements for centers that will provide such complex therapies.63
CONCLUSIONS
The treatment of advanced HF is rapidly changing, although many decisions are based more on consensus than on controlled clinical trials. The benefits obtained with ACE inhibitors, beta-blockers, resynchronization and implantable defibrillators regarding extended survival and the quality of life of patients with mild to moderate HF has given rise to a new population of patients with terminal HF who often present kidney failure and right ventricular failure. Mechanical assist devices offer a clear advantage compared to pharmacological treatment, but are still far from improving long-term life expectations. Heart transplant continues to be the only therapy that radically changes the natural evolution of these patients, with 1-year survival around 80% and 10-year survival around 50%, but this remains an opportunity for a very small number of patients.
Pharmacological treatment causes advanced terminal HF to evolve over a broad range of behaviors. These are characterized by a great variety of clinical stages, with periods of stability/instability whose duration is difficult to predict, and with uncertainty regarding the manner of death (sudden death or progression of circulatory congestion and low cardiac output) which exceeds that of any other terminal disease. Treatment for each patient should be individualized, ensuring that they understand their disease and that, in terminal situations, the physician can identify the preferences of the patient with regard to duration and quality of life, such that this helps in decision-making.
Section Sponsored by the Dr Esteve Laboratory
Correspondence: Dra. M.G. Crespo Leiro.
Servicio de Cardiología. Área del Corazón. Hospital Juan Canalejo.
Xubias, 84. 15006 A Coruña. España.
E-mail: MCreLei@canalejo.org