To the Editor,
Implanting a bioprosthesis using an endovascular approach, which avoids sternotomy and cardiopulmonary bypass, is becoming an alternative to conventional aortic valve replacement in patients at high surgical risk.1,2 Recently, some cases of bioprosthetic degeneration have been reported in which conventional reintervention in high-risk patients was avoided.3,4
We present the first case performed in Spain of transapical "valve-in-a-valve" implantation to treat a degenerated aortic valve bioprosthesis.
A woman aged 85 years, 45 kg, who underwent aortic valvular replacement with bovine pericardial bioprosthesis (21 mm Carpentier-Edwards Perimount) in August 1997, required sternal wire removal, debridement, and treatment during 1 year for granulomata and external fistulization.
After 9 years, echocardiographic monitoring showed progressive structural degeneration of the leaflets, and in October 2007 severe stenosis of the prosthesis was diagnosed. She was subsequently hospitalized 3 times due to heart failure and angina pectoris at rest; surgery was rejected due to the high risk. In February 2008, she was admitted for non-ST-segment acute myocardial infarction complicated by cardiogenic shock and mesenteric ischemia.
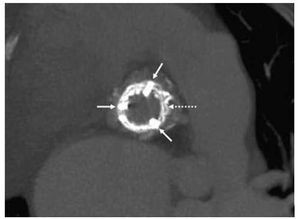
Figure 1. Axial view. The white arrows indicate the abutments and the Carpentier-Edwards bioprosthetic ring. The dotted line indicates the stent of the Edwards SAPIEN bioprosthesis inside the other bioprosthesis.
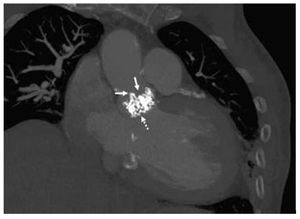
Figure 2. Coronal view. The white arrows indicate the abutments of the malfunctioning bioprosthesis. The dotted line points to the Edwards SAPIEN bioprosthetic valve inside the other bioprosthesis.
The echocardiogram showed severe calcification in the prosthetic leaflets leading to severe stenosis with a mean gradient of 64 mm Hg, moderate left ventricular hypertrophy, left ventricular ejection fraction of 38% and moderate-severe functional mitral regurgitation. Coronary angiography documented severe stenosis in the middle segment of the right coronary artery (80%). Computed angiotomography revealed "porcelain aorta" with severe atheromatosis from the root up to the femoral arteries. The common right iliac artery barely showed the filiform passage of contrast medium, and the left iliofemoral territory was severely affected: common iliac artery, 0.9-cm diameter; external iliac artery, 0.8 cm diameter; and femoral artery, 0.4 cm diameter.
Taking into account the clinical situation of the patient, the high surgical risk involved (logistic EuroSCORE, 33.27%; STS, 15.6%) and the difficulties involved in resternotomy due to previous mediastinal scarring, the choice of treatment was the implantation of a new bioprosthesis using the endovascular approach due to the iliofemoral anatomy described above.
The patient was operated in April 2009 by a multidisciplinary team of cardiac surgeons, cardiologists, and anesthesiologists, with radiological monitoring and transesophageal echocardiography. Following the technique described by Walther,5 a left anterior mini-thoractomy of 4 cm through the fifth intercostal space was performed. A temporary epicardial electrode was implanted and two purse strings sutures placed around the apex. A 0.035" guidewire was introduced by puncturing the apex and passing it through the bioprosthesis up to the descending aorta; this guidewire was then replaced by a stronger guidewire (Lunderquist Extra Straight Line Stiff Wire Guide, Cook Medical; Bloomington, Indiana, USA). Using overdrive pacing with a pacemaker at 180 beats/min, systolic blood pressure was reduced to below 80 mm Hg and valvuloplasty performed on the bioprosthesis. At the same time, a 23 mm Edwards SAPIEN bioprosthesis (Edwards Lifesciences, Irvine, California, USA) was mounted on a 26 Fr delivery system (Ascendra). The prosthesis was positioned at the ring of the degenerated bioprosthesis and, after new overpacing, the SAPIEN bioprosthesis was deployed using rapid inflation and deflation of the balloon. Once the implantation device was removed, the hemodynamic situation was reestablished, sutures closed and hemostasis achieved. Echocardiography and aortography confirmed the correct positioning and operation of the bioprosthesis, with no perivalvular regurgitation. The total duration of the procedure was 45 min.
After extubation in the operating theater, the patient remained in the recovery unit for 26 h and in the cardiology unit for 6 days without complications. Echocardiography showed improvement in left ventricular ejection fraction (50%) and mild mitral regurgitation. There was a mean transprosthetic gradient of 36 mm Hg and an effective prosthetic area of 1.3 cm2 (adjusted for body surface area, 0.89 cm2/m2).
In our view, the present case illustrates how the "valve-in-valve" technique is no more complex than that on a native valve and even has some advantages, such as improved fluoroscopic display during the procedure. This technique emerges as a therapeutic alternative in patients with bioprosthetic valve degeneration at very high surgical risk, porcelain aorta, patent mammary artery grafts, or a technically complex resternotomy.