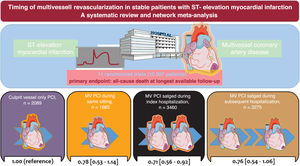
Multivessel percutaneous coronary intervention (MV-PCI) is recommended in patients with ST-segment elevation myocardial infarction (STEMI) and multivessel coronary artery disease (CAD) without cardiogenic shock. The present network meta-analysis investigated the optimal timing of MV-PCI in this context.
MethodsWe pooled the aggregated data from randomized trials investigating stable STEMI patients with multivessel CAD treated with a strategy of either MV-PCI or culprit vessel-only PCI. The primary outcome was all-cause death. The main secondary outcomes were cardiovascular death, myocardial infarction, and unplanned ischemia-driven revascularization.
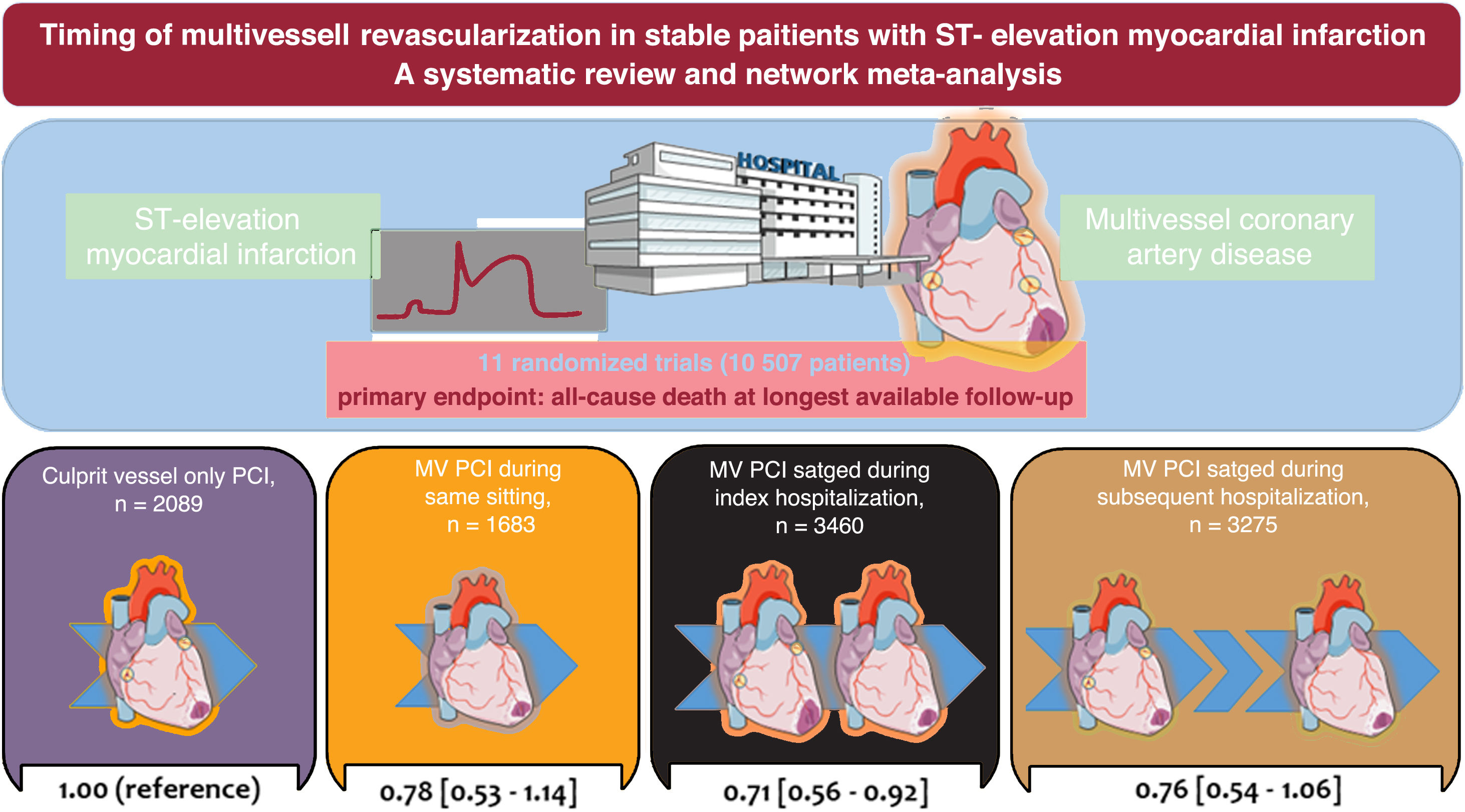
ResultsAmong 11 trials, a total of 10 507 patients were randomly assigned to MV-PCI (same sitting, n=1683; staged during the index hospitalization, n=3460; staged during a subsequent hospitalization within 45 days, n=3275) or to culprit vessel-only PCI (n=2089). The median follow-up was 18.6 months. In comparison with culprit vessel-only PCI, MV-PCI staged during the index hospitalization significantly reduced all-cause death (risk ratio, 0.73; 95%CI, 0.56-0.92; P=.008) and ranked as possibly the best treatment option for this outcome compared with all other strategies. In comparison with culprit vessel-only PCI, a MV-PCI reduced cardiovascular mortality without differences dependent on the timing of revascularization. MV-PCI within the index hospitalization, either in a single procedure or staged, significantly reduced myocardial infarction and unplanned ischemia-driven revascularization, with no significant difference between each other.
ConclusionsIn patients with STEMI and multivessel CAD without cardiogenic shock, multivessel PCI within the index hospitalization, either in a single procedure or staged, represents the safest and most efficacious approach. The different timings of multivessel PCI did not result in any significant differences in all-cause death.
This study is registered at PROSPERO (CRD42023457794).
Keywords
Approximately half of all patients with myocardial infarction (MI) have multivessel coronary artery disease (CAD), with at least 1 other significant stenosis in a nonculprit coronary vessel.1 Multivessel CAD in MI patients is associated with a higher risk of adverse cardiovascular events and mortality.2,3 For this reason, there is convincing evidence to recommend multivessel percutaneous coronary intervention (MV-PCI) in stable patients (ie, those without cardiogenic shock) with ST-segment elevation myocardial infarction (STEMI) and multivessel CAD.4 The recently released European guidelines for the management of patients with acute coronary syndrome (ACS) recommend a strategy of MV-PCI in STEMI patients presenting with multivessel CAD in the absence of cardiogenic shock.5 However, unlike previous European guidelines for the management of patients with STEMI,6 current guidelines suggest that MV-PCI can be performed either during the index procedure (same sitting) or staged during a subsequent hospitalization within 45 days of the index procedure.5
Recent randomized trials7–9 allow for a more comprehensive evaluation of the optimal timing of MV-PCI in patients with STEMI without cardiogenic shock. In contrast, previous observations did never focus on this specific topic, rather on the clinical superiority of MV-PCI as compared with a PCI of the culprit lesion only.10,11 Guidance of the multivessel treatment strategy in stable patients with STEMI has significant implications for clinical practice, encompassing logistical considerations and the diverse antithrombotic therapies employed in these patients. Therefore, we designed the present study to evaluate the clinical outcomes of patients with STEMI and multivessel CAD without cardiogenic shock according to the timing of MV-PCI.
METHODSData sources and outcomesWe searched electronic scientific databases, scientific abstracts of major cardiovascular conferences, and clinical trial registration websites from the inception of each database through August 2023 for randomized trials including hemodynamically stable STEMI patients with multivessel CAD undergoing either MV-PCI or culprit vessel-only PCI. All eligible studies were screened for further citations by reviewing the reference lists. Further information on the search strategy, study selection, data abstraction and quality assessment is provided in the supplementary methods (supplementary data).
The primary outcome was all-cause death. The main secondary outcomes were cardiovascular death, MI, and unplanned ischemia-driven revascularization. Other outcomes analyzed were major bleeding, stroke, and acute kidney injury. We included all endpoints occurring up to the maximum follow-up duration available as per definitions reported in the original trial protocols.
Data synthesis and statistical analysisBefore performing the statistical analysis, 2 investigators independently assessed the quality of each study using the Risk of Bias 2 (RoB 2) tool.12 Means for continuous variables and percentages for categorical variables were displayed as exploratory analyses for baseline features of participants enrolled in each included study. The weighted median follow-up duration was calculated based on the sample size of each individual study. Risk ratios (RR) with 95% confidence intervals (95%CI) and P values <.05 were used to compare outcomes of interest between treatment groups for all analyses.
First, we performed a frequentist network meta-analysis to generate direct and indirect evidence between interventions according to the definition of intention-to-treat analyses in the original trials. Second, we performed an exploratory pairwise meta-analysis, comparing a strategy of MV-PCI during the index hospitalization either in the same sitting or staged (MV-PCI during the index hospitalization group) vs a strategy of MV-PCI during a subsequent hospitalization within 45 days or a culprit vessel-only PCI (control group). Further details concerning the statistical framework of the network and pairwise meta-analyses are reported in the supplementary methods (supplementary data).
This study is reported in compliance with the PRISMA statement (table 1 of the supplementary data).13 All analyses were performed using the packages netmeta, meta, and metafor with R version 4.1.3 (R Foundation for Statistical Computing, Austria). No extramural funding was used to support this work. This study did not require ethics approval. The study is registered at PROSPERO (CRD42023457794). The data supporting the findings of this study are available from the corresponding author upon reasonable request.
RESULTSEligible and included studiesThe flow diagram for the trial selection process is shown in figure 1 of the supplementary data. After application of the inclusion/exclusion criteria, 11 trials, all published as full-length manuscripts,7–9,14–21 were included in the meta-analysis. There was no disagreement requiring consultation with a third reviewer. In the selected trials, a total of 10 507 patients with MI and multivessel CAD were randomly assigned to MV-PCI (same sitting, n=1683; staged during the index hospitalization, n=3460; staged during a subsequent hospitalization within 45 days, n=3275) or to culprit vessel-only PCI (n=2089). The main characteristics of the included trials are shown in table 1. All except 1 trial15 had a multicenter design and predominantly included patients with stable STEMI and multivessel CAD with an indication for PCI. The BioVasc trial7 and the FIRE trial9 included patients with STEMI or non-STEMI. In addition, the BioVasc trial included 127 patients with unstable angina (1.2% of the overall population included in this analysis).
Main features of the trials included in the study
| Trial | Multicenter | Enrolment period | Comparison | Main inclusion criteria | Definition of multivessel CAD | Primary endpoint | Available follow-up (months) | |
|---|---|---|---|---|---|---|---|---|
| BioVasc7 | Yes | June 2018-October 2021 | Same sitting MV-PCI (n=764) | Staged MV-PCI (subsequent, n=761) | Age 18-85 y; STEMI or NSTEACS; multivessel CAD with a clearly identifiable culprit lesion | ≥ 2 or more coronary arteries with a diameter ≥ 2.5mm and ≥ 70% stenosis by visual estimation or positive coronary physiology testing | All-cause death, any MI, unplanned ischemia-driven revascularization, stroke | 12 |
| CompareAcute20 | Yes | July 2011-October 2015 | Staged MV-PCI (index, n=295)* | Culprit vessel-only PCI (n=590) | Age 18-85 y; STEMI; ≤ 12 h after symptom onset; indication for primary PCI; multivessel CAD deemed appropriate for PCI by the interventional cardiologist | Culprit artery plus nonculprit artery with ≥ 50% angiographic diameter stenosis | All-cause death, nonfatal MI, any revascularization, stroke | 12 |
| COMPLETE21 | Yes | February 2013-March 2017 | Staged MV-PCI (index, n=2016)* | Staged MV-PCI (subsequent, n=2025) | STEMI; multivessel CAD | > 1 angiographically significant (≥ 70% stenosis of the vessel diameteron visual estimation or> 50 to 69% stenosisaccompanied by a fractional flow reservemeasurement of ≤ 0.80) nonculprit artery amenable to successful PCI, located in a vessel with a diameter ≥ 2.5mm and not stented during the index PCI of culprit artery | Cardiovascular death or reinfarction; cardiovascular death, reinfarction, or ischemia-driven revascularization | 36 |
| CvLPRIT18 | Yes | May 2011-May 2013 | Same sitting MV-PCI (n=150) | Culprit vessel-only PCI (n=146) | Suspected or proven MI (angiographic confirmation of culprit artery occlusion); <12 h after symptom onset; eligible for primary PCI; multivessel CAD | Culprit artery plus ≥ 1 nonculprit artery (main branch or side branch) with ≥ 1 angiographically significant lesion (> 50% diameter stenosis in 2 planes or> 70% in 1 plane); ≥ 2mm diameter suitable for stent implantation | All-cause death, recurrent MI, heart failure, ischemia-driven revascularization by PCI/CABG | 66 |
| DANAMI-3-PRIMULTI17 | Yes | March 2011-February 2014 | Staged MV-PCI (index, n=314)* | Culprit vessel-only PCI (n=313) | Chest pain <12 h after symptom onset and ST-segment elevation> 0.1mV in ≥ 2 contiguous leads; successful primary PCI of the culprit lesion (defined TIMI flow ≥ 2 and <30% residual stenosis); multivessel CAD | ≥ 1 nonculprit artery with> 50% angiographic diameter stenosis | All-cause death, recurrent MI, ischemia-driven revascularization of nonculprit artery | 27 |
| FIRE9 | Yes | July 2019-October 2021 | Staged MV-PCI (index, n=720)* | Culprit vessel-only PCI (n=725) | Age ≥ 75 y; STEMI or NSTEMI with successful PCI of the culprit lesion; multivessel CAD | ≥ 1 nonculprit lesion, located in a vessel with a diameter ≥ 2.5mm and a visually estimated diameter stenosis of ≥ 50 to 99% | Death, MI, stroke, or ischemia-driven coronary revascularization | 12 |
| Hamza M, et al.19 | Yes | June 2013-February 2014 | Staged MV-PCI (index, n=50) | Staged MV-PCI (subsequent, n=50) | Diabetes mellitus; STEMI <12 h after symptom onset; eligible for primary PCI; multivessel CAD | Culprit artery plus nonculprit artery with ≥ 80% angiographic diameter stenosis | All-cause death; recurrent and ischemia-driven revascularization | 6 |
| HELP AMI14 | Yes | N/R | Same sitting MV-PCI (n=52) | Staged MV-PCI (subsequent, n=17) | Chest pain <12 h before hospital admission; ST-segment elevation> 0.1mV in ≥ 2 contiguous leads (peripheral) or> 0.2mV in ≥ 2 contiguous precordial leads; multivessel CAD | Culprit artery plus 1-3 lesions in a major nonculprit artery | Repeat revascularization | 12 |
| MULTISTARS AMI8 | Yes | October 2016-June 2022 | Same sitting MV-PCI (n=418) | Staged MV-PCI (subsequent, n=422) | STEMI <24 h after symptom onset; eligible for primary PCI; multivessel CAD | Stenosis ≥ 70% in at least 2 projections in nonculprit artery with a lumen diameter ≥ 2.25 and ≤ 5.75 mm | All-cause death, nonfatal MI, stroke, unplanned ischemia-driven revascularization, hospitalization for heart failure | 12 |
| Politi L, et al.15 | No | January 2003-December 2007 | Same sitting MV-PCI (n=65)/ Staged MV-PCI (index, n=65) | Culprit vessel-only PCI (n=84) | Chest pain lasting> 30min and <12 h before hospital admission; ST-segment elevation> 0.1mV in ≥ 2 contiguous leads (peripheral) or> 0.2mV in ≥ 2 contiguous precordial leads; multivessel CAD | > 70% stenosis of ≥ 2 coronary arteries or major branches by visual estimation | All-cause death, in-hospital death, reinfarction, rehospitalization for ACS, repeat coronary revascularization by PCI/CABG | 30 |
| PRAMI16 | Yes | April 2008-January 2013 | Same sitting MV-PCI (n=234) | Culprit vessel-only PCI (n=231) | STEMI; multivessel CAD | ≥ 1 nonculprit artery with ≥ 50% stenosis deemed to be treated by PCI | All-cause death, any MI, unplanned ischemia-driven revascularization, stroke | 23 |
CABG, coronary artery by-pass graft; CAD, coronary artery disease; MI, myocardial infarction; MV-PCI, multivessel percutaneous coronary intervention; N/A, not applicable; N/R, not reported; NSTEACS, non–ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
In the multivessel PCI group, the revascularization of nonculprit lesions was mostly staged during the index hospitalization. In the COMPLETE trial21, the largest trial among those included in the present analysis, the operators were asked to specify whether they intended to perform a PCI of nonculprit lesions during the index hospitalization or in a subsequent hospitalization in case the patients were to be assigned to a MV-PCI strategy.22 One trial had a 3-arm design.15 In 4 out of 11 trials,9,17,20,21 the decision to perform PCI of nonculprit lesions in the MV-PCI group was guided by physiology, while in the remaining trials the decision was based on angiographic criteria of lesion severity. In 8 trials,7–9,16–18,20,21 prasugrel and ticagrelor were used in combination with aspirin as the dual antiplatelet therapy regimen for a period ranging between 6 and 12 months.
The baseline characteristics of the patients included in the original trials are shown in table 2. The median age was 64.4 [interquartile range, 62-65] years, more than two-thirds of the patients were male, roughly one-fifth had type 2 diabetes, and nearly 50% of them had hypertension at the time of enrolment in the primary trials. Almost half of the patients were current or former smokers, and a prior MI was reported in less than 10% of participants. Approximately 40% of the included patients presented with anterior MI. The weighted median follow-up available for this analysis was 18.6 months.
Main characteristics of the patients enrolled among the trials included in the study
| Trial | Patients, n | Age, years | Male, % | Diabetes mellitus, % | Hypertension, % | Smoking, % | Prior MI, % | Anterior MI, % |
|---|---|---|---|---|---|---|---|---|
| BioVasc7 | 1525 | 65.5 | 77.8 | 11.0 | 53.6 | 51.6 | 10.3 | 36.5 |
| CompareAcute20 | 885 | 61.5 | 77.1 | 15.4 | 47.2 | 46.1 | 7.9 | 35.1 |
| COMPLETE21 | 4041 | 62.0 | 79.8 | 19.5 | 49.7 | 39.7 | 7.4 | 34.3 |
| CvLPRIT18 | 296 | 64.8 | 81.0 | 13.6 | 36.6 | 30.6 | 4.0 | 35.8 |
| DANAMI-3-PRIMULTI17 | 627 | 63.5 | 81.1 | 11.3 | 44.0 | 49.6 | 7.0 | 34.6 |
| FIRE9 | 1445 | 80.5 | 63.4 | 32.0 | 82.0 | 8.5 | 15.2 | 45.6 |
| Hamza M, et al.19 | 100 | 54.3 | 84.0 | 50.0 | 31.0 | 75.0 | 8.0 | 47.0 |
| HELP AMI14 | 69 | 64.4 | 87.0 | 18.8 | 42.0 | 71.0 | N/R | 53.6 |
| MULTISTARS AMI8 | 840 | 65.0 | 87.8 | 15.6 | 52.3 | 50.8 | 5.7 | 40.4 |
| Politi L, et al.15 | 214 | 65.3 | 77.5 | 19.1 | 57.9 | N/R | N/R | 44.0 |
| PRAMI16 | 465 | 62.0 | 78.0 | 17.8 | 40.2 | 51.8 | 7.5 | 33.5 |
MI, myocardial infarction; N/R, not reported; PCI, percutaneous coronary intervention.
Overall proportions and means are reported. The column headed “Prior MI” presents the proportion of patients with previous myocardial infarction. The column headed “Anterior MI” presents the proportion of patients with anterior myocardial infarction at the time of the index procedure.
All trials had sufficient statistical power for composite clinical outcomes, which included all-cause death, MI, and/or unplanned ischemia-driven revascularization in most cases. Four trials had available outcomes data beyond 24 months.15,17,18,21 Outcome definitions are reported in table 2 of the supplementary data, and the Risk of Bias interstudy is presented in figure 2 of the supplementary data. All patients were available for assessment of outcomes of interest. The complete ranking of revascularization strategies for each outcome of interest is reported in the table 3 of the supplementary data.
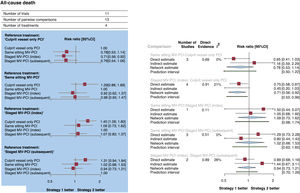
Primary outcomeThe network of treatment strategies for all-cause death, the primary outcome, is reported in figure 3 of the supplementary data. All-cause death occurred in 528 patients (5.0%). Compared with culprit vessel-only PCI, a strategy of MV-PCI staged during the index hospitalization significantly reduced all-cause death (RR, 0.73; 95%CI, 0.56-0.92; P=.008), whereas a strategy of MV-PCI performed during the same sitting (RR, 0.78; 95%CI, 0.53-1.14) or staged during a subsequent hospitalization within 45 days did not (RR, 0.76; 95%CI, 0.54-1.06). These results are reported in the figure 1 and figure 2A,B. The results remained consistent after considering the different follow-up durations of the included studies. Compared each other, MV-PCI strategies showed no significant differences or statistical heterogeneity (I2=0%). MV-PCI staged during the index hospitalization ranked as possibly the best option to prevent all-cause death compared with all other strategies (P=.78). The node-splitting method did not reveal significant disagreement between direct and indirect evidence (figure 2B and table 4 of the supplementary data). A small-study effect and a significant publication bias could be rejected both visually (figure 4 of the supplementary data) and statistically (P=.41). The league of risk estimates for the primary and the secondary outcomes from network meta-analysis is reported in the table 5 of the supplementary data.
Forest plots. A: forest plot from network meta-analysis for all-cause death. The forest plot of pooled risk ratios and 95%CI for all-cause death were derived from a network meta-analysis. B: forest plot from node-split model analysis for all-cause death. The forest plots of pooled risk ratios and 95%CI for all-cause death were derived from a node-splitting analysis of inconsistency between accumulated direct and indirect evidence. The number under the heading “direct evidence” indicates the proportion of direct evidence within the network estimate. 95%CI, 95% confidence interval; MV-PCI, multivessel percutaneous coronary intervention; PCI, percutaneous coronary intervention; RR, risk ratio.
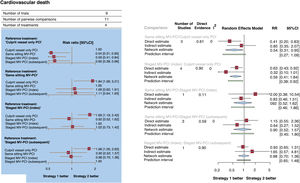
Cardiovascular death occurred in 292 patients (3.0%, data available for 9780 patients; figure 3A,B). Compared with culprit vessel-only PCI, all MV-PCI strategies significantly reduced cardiovascular death: same sitting (RR, 0.54; 95%CI, 0.31-0.95; P=.03); staged during the index hospitalization (RR, 0.59; 95%CI, 0.41-0.84; P=.004); and staged during a subsequent hospitalization within 45 days (RR, 0.60; 95%CI, 0.38-0.96; P=.03). Compared each other, MV-PCI strategies showed no significant differences or statistical heterogeneity (I2=0%). MV-PCI during the same sitting (P=.75) ranked as possibly the best option to prevent cardiovascular death.
Forest plots. A: forest plot from network meta-analysis for cardiovascular death. The forest plots of pooled risk ratios and 95%CI for cardiovascular death were derived from a network meta-analysis. B: Forest plot from node-split model analysis for cardiovascular death. The forest plots of pooled risk ratios and 95%CI for cardiovascular death were derived from a node-splitting analysis of inconsistency between accumulated direct and indirect evidence. The number under the heading “direct evidence” indicates the proportion of direct evidence within the network estimate. 95%CI, 95% confidence interval; MV-PCI, multivessel percutaneous coronary intervention; PCI, percutaneous coronary intervention; RR, risk ratio.
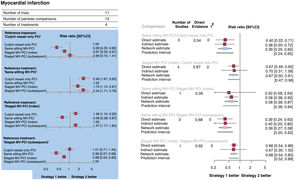
MI occurred in 559 patients (5.3%; figure 4A,B). Compared with culprit vessel-only PCI, a strategy of MV-PCI during the same sitting (RR, 0.39; 95%CI, 0.25-0.60; P <.001) or staged during the index hospitalization (RR, 0.67; 95%CI, 0.50-0.91; P=.001) significantly reduced MI, whereas a strategy of MV-PCI staged during a subsequent hospitalization within 45 days did not (RR, 0.99; 95%CI, 0.70-1.41). Compared each other, a strategy of MV-PCI during the same sitting was superior to a strategy of MV-PCI staged during the index hospitalization (RR, 0.58, 95%CI, 0.38-0.87; P=.009) and to a strategy of MV-PCI staged during a subsequent hospitalization within 45 days (RR, 0.39; 95%CI, 0.27-0.58; P <.001). There was no statistical heterogeneity (I2=0%). MV-PCI during the same sitting (P=.99) ranked as possibly the best option to prevent MI.
Forest plots. A: forest plot from network meta-analysis for myocardial infarction. The forest plots of pooled risk ratios and 95%CI for myocardial infarction are derived by network meta-analysis. B: forest plot from node-split model analysis for myocardial infarction. The forest plots of pooled risk ratios and 95%CI for myocardial infarction are derived by a node-splitting analysis of inconsistency between cumulated direct and indirect evidence. The number under the label “direct evidence” describes the proportion of direct evidence within the network estimate. 95%CI, 95% confidence interval; MV-PCI, multivessel percutaneous coronary intervention; PCI, percutaneous coronary intervention; RR, risk ratio.
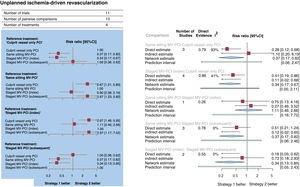
Unplanned ischemia-driven revascularization occurred in 872 patients (8.3%; figure 5A,B). In comparison with culprit vessel-only PCI, a strategy of MV-PCI during the same sitting (RR, 0.37; 95%CI, 0.17-0.82; P=.015) or staged during the index hospitalization (RR, 0.34; 95%CI, 0.17-0.67; P=.002) significantly reduced unplanned ischemia-driven revascularization, whereas a strategy of MV-PCI staged during a subsequent hospitalization within 45 days did not (RR, 1.00; 95%CI, 0.38-2.62). Compared each other, the MV-PCI strategies showed no significant differences, but did show significant statistical heterogeneity (I2=85.3%). The node-split analysis revealed that the significant inconsistency between direct and indirect estimates was largely attributable to the comparison between same sitting MV-PCI and culprit vessel-only PCI. MV-PCI staged during the index hospitalization (P=.86) ranked as possibly the most effective options to prevent unplanned ischemia-driven revascularization.
Forest plots. A: Forest plot from a network meta-analysis for unplanned ischemia-driven revascularization. The forest plots of pooled risk ratios and 95%CI for unplanned ischemia-driven revascularization were derived from a network meta-analysis. B: forest plot from node-split model analysis for unplanned ischemia-driven revascularization. The forest plots of pooled risk ratios and 95%CI for unplanned ischemia-driven revascularization were derived from a node-splitting analysis of inconsistency between accumulated direct and indirect evidence. The number under the heading “direct evidence” indicates the proportion of direct evidence within the network estimate. 95%CI, 95% confidence interval; MV-PCI, multivessel percutaneous coronary intervention; PCI, percutaneous coronary intervention; RR, risk ratio.
Major bleeding occurred in 252 patients (2.6%, data available for 9759 patients). The revascularization strategies showed no significant differences or statistical heterogeneity (I2=9%) for this outcome. MV-PCI during the same sitting (P=.82) ranked as possibly the most effective option to prevent major bleeding (figure 5 of the supplementary data).
Stroke occurred in 134 patients (1.4%, data available for 9759 patients). The revascularization strategies showed no significant differences for this outcome or statistical heterogeneity (I2=0%). A culprit vessel-only PCI (P=.70) and a same sitting MV-PCI (P=.69) ranked as possibly the most effective options to prevent stroke.
Acute kidney injury occurred in 353 patients (4.4%, data available for 8028 patients). Compared with culprit vessel-only PCI, the revascularization strategies showed no significant differences or statistical heterogeneity (I2=9%) for this outcome. Of note, compared with a strategy of MV-PCI staged during the index hospitalization, MV-PCI staged during a subsequent hospitalization within 45 days reduced significantly acute kidney injury (RR, 0.58; 95%CI, 0.35-0.97; P=.040) and ranked as possibly the most effective options to prevent this adverse outcome (P=.84).
Sensitivity analyses and pairwise meta-analysisThe network meta-analysis was restricted to trials that used angiography alone to guide MV-PCI (figure 6A of the supplementary data), trials in which more potent P2Y12-inhibitors were prescribed (figure 6B of the supplementary data), and trials with more stringent criteria for defining multivessel CAD (figure 6C of the supplementary data). These analyses did not reveal a significant change in the direction of risk estimates for the primary outcome. The network meta-analysis restricted to trials that included only patients with STEMI (figure 7A of the supplementary data) or to trials enrolling >500 participants (figure 7B of the supplementary data) did not reveal a significant change in the direction of risk estimates for the primary outcome. However, the differences previously observed in the overall analysis were no longer statistically significant.
In the pairwise meta-analysis (figure 8 of the supplementary data), 236 deaths (4.6%) were reported in patients assigned to MV-PCI during the index hospitalization and 292 deaths (5.4%) were reported in patients assigned to the control strategy (RR, 0.85, 95%CI, 0.72-1.00; P=.050). There was no evidence of statistical heterogeneity, and the prediction interval crossed the null. Of interest, we found a nearly 3% absolute risk reduction for MI and a 13% absolute risk reduction for unplanned ischemia-driven revascularization in patients assigned to MV-PCI during the index hospitalization compared with patients assigned to control strategy (figure 9 of the supplementary data).
DISCUSSIONThis meta-analysis combined study-level data from more than 10 000 MI patients with multivessel CAD without cardiogenic shock who were randomly assigned to either a strategy of MV-PCI at different time points or culprit vessel-only PCI. The primary conclusions of this meta-analysis are as follows: a) A strategy of MV-PCI vs culprit vessel-only PCI reduces cardiovascular mortality, irrespective of the timing chosen for the completion of revascularization. b) A strategy of MV-PCI during the index hospitalization, whether performed in a single sitting or staged, significantly reduces MI and unplanned ischemia-driven revascularization and was identified as the most favorable option in terms of safety and efficacy. c) The different timings of multivessel PCI did not result in any significant differences in all-cause mortality.
To the best of our knowledge, this is the first meta-analysis investigating the timing of MV-PCI in STEMI patients with multivessel CAD without cardiogenic shock. By including the recently published BioVasc, MULTISTARS AMI, and FIRE trials,7–9 this study offers the broadest updated evidence on the topic. Unlike a recent analysis,23 the present study included predominantly patients with STEMI, allowing more reliable identification of culprit and nonculprit lesions. Overall, the results of the present study deserve careful consideration.
First, this meta-analysis explored a possible difference in mortality between different timings of MV-PCI in hemodynamically stable patients with STEMI. Previous meta-analyses have evaluated the role of multivessel revascularization in patients with stable MI and multivessel CAD, while the optimal timing of multivessel PCI in this setting has been less studied. In one study,10 a subgroup analysis reported fewer cardiovascular deaths with MV-PCI, irrespective of whether the revascularization was performed during the same sitting or staged. Another study reported a significant modification of the treatment effect for the composite outcome of cardiovascular death and reinfarction by performing same-sitting MV-PCI.24 This latter advantage was mostly attributable to fewer reinfarction events. Another subanalysis in approximately 2000 patients showed a significant relative risk reduction for death with MV-PCI performed during the index hospitalization.11
In contrast, a subgroup analysis of the COMPLETE trial demonstrated that the superiority of MV-PCI over culprit vessel-only PCI in terms of cardiovascular death and MI was independent of whether the PCI of nonculprit lesions was performed during the index hospitalization or in a subsequent hospitalization within 45 days.22 Unfortunately, the timing of PCI was not a random factor, and same-sitting MV-PCI was not a comparator. Therefore, the interpretation of the results from that analysis is subject to a substantial bias that cannot be completely accounted for. In contrast, in comparison with a strategy of culprit vessel-only PCI, the present study found fewer deaths from any cause with a strategy of MV-PCI during the index hospitalization and fewer cardiovascular deaths with any MV-PCI strategy, regardless of the timing chosen for completion of revascularization. This finding is reassuring and less subject to bias, given the large number of patients available and the robust statistical support.
Second, we report a reduction in MI in the group of patients assigned to MV-PCI at any time during the index hospitalization compared with culprit vessel-only PCI. Of note, although this finding is not novel, there was a change in the magnitude of treatment effect depending on the revascularization of nonculprit lesions during the same sitting, owing to a 30% incremental relative risk reduction for MI. This result could be partially attributable to a detection bias, with procedure-related MI being less frequently diagnosed in patients undergoing MV-PCI in the context of pre-existing elevated cardiac markers due to the acute MI event. On the other hand, the concept of ACS as a systemic disease involving the entire coronary tree beyond the culprit coronary vessel has been confirmed in several imaging studies showing the unstable nature of nonculprit lesions at increased risk for plaque rupture and subsequent thrombotic events.25 In this regard, a strategy of MV-PCI during the index hospitalization (either in the same sitting or staged) could help prevent adverse cardiovascular events due to vulnerable nonculprit lesions.26 In support of this argument, it is worth mentioning that the observed reduction in MI with a strategy of MV-PCI compared with a strategy of culprit vessel-only PCI disappeared with a strategy of MV-PCI staged during a subsequent hospitalization within 45 days.
Last, we observed a clinically relevant relative risk reduction for unplanned ischemia-driven revascularization in patients undergoing MV-PCI during the index hospitalization (either in the same sitting or staged) compared with patients treated with culprit vessel-only PCI, while a strategy of MV-PCI staged during a subsequent hospitalization within 45 days did not show a meaningful clinical impact. Although this result is clinically plausible, we found wide statistical heterogeneity. From a scientific standpoint, the open-label design of the trials and the different thresholds to perform unplanned ischemia-driven revascularizations in the culprit vessel-only PCI group is likely to play a role. This fact lends further support to the selection of all-cause death as a primary endpoint in such analyses. Of note, recently released European guidelines for the management of patients with ACS recommend that the decision to revascularize nonculprit lesions in STEMI patients with multivessel CAD without cardiogenic shock should rely on angiographic severity and discourage a physiological assessment of nonculprit vessels during the index procedure.5 Arguably, the role of intravascular imaging during the index PCI to assess the degree of a bystander stenosis and to stratify the nature of nonculprit lesions is expected to increase in the coming years and will be subject to further investigation, in accordance with data suggesting that nearly half of subsequent thrombotic events in patients with ACS are associated with atherosclerotic disease progression in remote coronary segments or vessels.26,27
LimitationsThis study has some limitations. First, this is a study-level meta-analysis, and a meta-analysis of individual participants remains the gold standard. Although the subgroup analysis did not reveal a change in the magnitude of the treatment effect for the primary outcome, this study cannot fully explore the impact of more potent, contemporary antithrombotic regimens on the timing of MV-PCI. In the same vein, the different timing of revascularization also implies a distinct use of antithrombotic drugs, in terms of initiation and duration. Unfortunately, the unavailability of patient-level data does not allow the analysis to be adjusted for these characteristics.
Second, in the pairwise meta-analysis, the control group consisted of patients in which nonculprit lesions were either managed with optimal medical therapy or in a staged procedure within 45 days of hospital discharge. It was determined that combining the participants from these groups into the control group was clinically appropriate, given that previous evidence suggested that, in the early postrandomization phase, patients assigned to a staged PCI after discharge or to medical management of nonculprit coronary lesions have a comparable risk for nonfatal MI and unplanned ischemia-driven revascularization.7,8,18
Third, with regard to the results of this analysis, it should be noted that they do not apply to patients with clinical and anatomical features other than those presented here. All trials included in this analysis excluded patients with cardiogenic shock, and randomization was restricted to patients undergoing successful treatment of culprit vessels. Women were largely underrepresented in all the trials. In addition, the presence of a chronic total occlusion was a main exclusion criterion in 7 trials.7,8,16–20
Lastly, approximately 15% of the population included had NSTEMI. Although the magnitude and direction of the treatment effect for the primary outcome did not change according to the type of MI, further investigation is required to confirm that in patients with NSTEMI, an index hospitalization strategy of MV-PCI reduces the risk of adverse cardiovascular events.28
CONCLUSIONSIn patients with STEMI and multivessel CAD without cardiogenic shock, a strategy of multivessel PCI during the index hospitalization, either in the same sitting or staged, is associated with overall improved outcomes compared with other treatment strategies. Therefore, complete revascularization during the index hospitalization should become the treatment of choice in patients with STEMI and multivessel CAD without cardiogenic shock.
FUNDINGThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ETHICAL CONSIDERATIONSThe present study is a meta-analysis of aggregate data and did not require ethics approval or patient recruitment or access to disaggregated information on individuals. Possible sex/gender biases were taken into account in the preparation of this paper.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this article.
AUTHORS’ CONTRIBUTIONSF. Voll, A. Kastrati and S. Cassese were involved in study conception and design. S. Cassese performed the data analysis. A. Kastrati supervised the data analysis. F. Voll, together with G. Ndrepepa, A. Kastrati and S. Cassese, wrote the first draft of the manuscript. C. Kuna, M. Scalamogna, T. Kessler, S. Kufner, T. Rheude, H.B. Sager, E. Xhepa, J. Wiebe, M. Joner, H. Schunkert, G. Ndrepepa, B.E. Stähli, A. Kastrati and S. Cassese were involved in data acquisition and revised the manuscript for important intellectual contents. All authors had full access to all the data, including statistical reports and tables and approved the manuscript for final submission.
CONFLICTS OF INTERESTC. Kuna has received speaker fees from AstraZeneca. S. Kufner has received speaker and consulting fees from AstraZeneca, Bristol-Myers Squibb, and Translumina. T. Rheude has received speaker fees from AstraZeneca and SIS Medical AG. M. Joner reports institutional grant support from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences and Infraredx; consulting fees from Biotronik, TriCares, Veryan, and Shockwave; speaker fees from Abbott, AstraZeneca, Biotronik, Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, Recor Medical and Shockwave; participation on a Steering Committee of Biotronik and Edwards Lifesciences; travel support from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences and SIS Medical AG. E. Xhepa has received lecture fees/honoraria from AstraZeneca, Boston Scientific and SIS Medical AG; and proctoring honoraria from Abbott Vascular; and institutional grant support from Abbott Vascular. B.E. Stähli has been supported by the H.H. Sheikh Khalifa bin Hamad Al-Thani Research Programme, and reports research grants to the institution from Boston Scientific, the B. Braun Foundation, the German Center for Cardiovascular Research (DZHK), the German Heart Research Foundation, Edwards Lifesciences, the Iten-Kohaut Foundation, and the OPO Foundation, and consulting and speaker fees from Abbott Vascular, Abiomed, Boston Scientific, and SMT. S. Cassese has received lectures/proctoring honoraria from AstraZeneca, SIS Medical AG and Translumina; and institutional grant support from Abbott Vascular, Boston Scientific and SIS Medical AG. The other authors declare no potential conflict of interest.
- –
In patients with STEMI and multivessel CAD without cardiogenic shock, multivessel PCI is superior to culprit vessel-only PCI. The optimal timing of the multivessel PCI remains a matter of debate, with some advocating for its performance during the index procedure, while others suggest scheduling it during a subsequent hospitalization.
- –
In patients with STEMI and multivessel CAD without cardiogenic shock, multivessel PCI during the index hospitalization, either in the same sitting or staged, reduces the risk of death, MI, and unplanned repeat revascularization and should represent the strategy of choice in this setting.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2024.06.002