To compare the 3-year incidence of major events in patients with bifurcation lesions treated with provisional sirolimus-eluting stents vs everolimus-eluting stents.
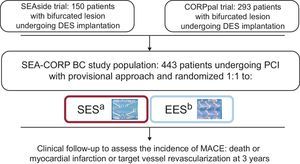
MethodsA pooled analysis of 2 prospective randomized trials with similar methodology (SEAside and CORpal) was performed. In these trials, 443 patients with bifurcation lesions were randomly assigned to treatment with either sirolimus-eluting stents or everolimus-eluting stents. The clinical follow-up was extended up to 3 years to assess major adverse cardiovascular events (death or acute myocardial infarction or target vessel revascularization).
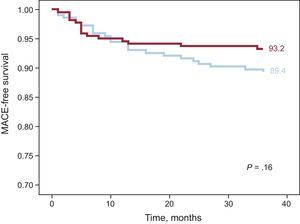
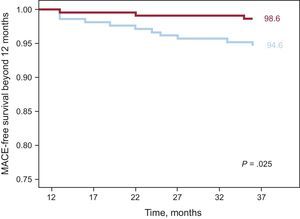
ResultsAt 3 years, survival free of major adverse cardiovascular events was 93.2% vs 91.3% in the everolimus-eluting stent group vs the sirolimus-eluting stent group (P = .16). Exploratory land-mark analysis for late events (occurring after 12 months) showed significantly fewer major adverse cardiovascular events in the everolimus-eluting stent group: 1.4% vs 5.4% in the sirolimus-eluting stent group (P = .02).
ConclusionsProvisional stenting with either sirolimus-eluting stents or everolimus-eluting stents in bifurcation lesions is associated with low rates of major adverse events at 3-years’ follow-up. The results of a subanalysis of events beyond 1 year, showing a lower event rate with everolimus-eluting stents than with sirolimus-eluting stents, suggest that studies exploring the long-term clinical benefit of the latest generation of drug-eluting stents are warranted.
Keywords
Provisional side branch (SB) stenting is the currently recommended percutaneous approach for the treatment of patients with coronary bifurcation lesions.1 However, their outcome may be influenced by the type of drug-eluting stents (DES) implanted in the main vessel (MV). Indeed, during the treatment of a bifurcation, both the metal platform of the stent may be deformed and the polymer damaged.2 Moreover, blood flow turbulences occurring at the level of stents implanted across bifurcations may facilitate both restenosis and thrombotic events. Accordingly, specific clinical trials in patients with bifurcation lesions need to be designed to establish differences among currently available DES.
This article is the result of a cooperative study to compare the efficacy and safety between 2 of the most widely used DES. We performed a pooled analysis of the 2 available prospective randomized trials with similar methodology (SEAside and CORpal)3,4 with a clinical follow-up extended up to 3 years. The primary objective of this study was to analyze differences in major adverse cardiovascular events (MACE) during this period between the groups of patients treated with an sirolimus-eluting stent (SES) or an everolimus-eluting stent (EES).
METHODSThe StudiesThis pooled analysis, the SEAside study, included 150 patients with bifurcation lesions undergoing DES implantation using a provisional stenting strategy. The patients were randomized to receive an SES (n = 75) or an EES (n = 75) before the intervention. The diameter of the MV and SB were required to be ≥ 2.5 mm and ≥ 2.0 mm, respectively, by visual estimation; data collected at the 18-month follow-up have previously been reported.3 The investigators in the CORpal study randomly assigned 293 patients with bifurcation lesions to treatment with either an SES (n = 145) or an EES (n = 148). The diameters of the MV and SB were required to be ≥ 2.50 mm and ≥ 2.25 mm, respectively, by visual estimation. The 1-year follow-up has also previously been reported.4
PatientsDuring the years 2007 and 2008, 443 patients with bifurcation lesions treated with provisional SB stenting from 3 centers (2 in Spain and 1 in Italy) were recruited and randomly assigned to receive an SES (Cypher Select, Cordis; Warren, New Jersey, United States) or an EES (Xience V, Abbott Vascular, Santa Clara, California, United States) at the MV. The cooperative study flowchart is summarized in Figure 1. Patients fulfilled the following inclusion and exclusion criteria: a) the lesion was > 50% and located in a major bifurcation point, regardless of the length, morphology, or angulation; b) the ≥ 2.50mm in diameter; c) the SB was ≥ 2.00 mm in diameter, in the SEAside study and > 2.25mm in the CORpal study, and d) the SB stenosis length was < 10mm in the CORpal study, while no limitations were made in the SEAside concerning SB lesion length. The exclusion criteria were as follows: a) contraindications to prolonged dual antiplatelet therapy; b) acute phase of myocardial infarction (direct or rescue angioplasty). In the SEAside trial, patients had to have no acute (within 48h) ST-segment elevation acute myocardial infarction, while researchers, from the CORpal trial included patients with an acute myocardial infarction after 24h of intravenous thrombolysis and c) cardiogenic shock. Written informed consent was obtained from all patients.
ProcedurePercutaneous coronary interventions were performed by the radial or femoral approach according to the physician's preference. All patients were treated with stents using a simple approach or provisional SB stenting. Thus, a first stent was implanted at the MV, leaving a wire at the SB that became jailed between the metallic structure of the stent and the vessel wall. At this point, the SB ostium was evaluated. If there was SB compromise, a simultaneous or sequential SB postdilation was performed. After this maneuver, the SB ostium was evaluated again and a second stent was implanted in the SB if deemed necessary by the operator in the SEAside study and if there was a residual stenosis > 50% or a coronary TIMI (Thrombolysis in Myocardial infarction) flow < 3 flow in the CORpal study. SB stenting was performed according to the T-stenting technique.5 Procedural success was defined as TIMI flow grade 3 in both the MV and the SB and visual residual stenosis ≤ 20% in the MV. At the time of the percutaneous coronary intervention, all patients were on dual antiplatelet therapy with acetylsalicylic acid and thienopyridines. Procedural anticoagulation was achieved with unfractionated heparin (70-100 U/kg intravenous bolus with further dose adjustment to maintain an activated clotting time of approximately 300s). In both studies, the use of glycoprotein IIb/IIIa inhibitors was at the operator's discretion. After the procedure, all patients received dual antiplatelet therapy for at least 12 months with instructions to continue acetylsalicylic acid indefinitely. After the percutaneous coronary intervention, all patients underwent a postpercutaneous coronary intervention electrocardiogram and 6- and 24-h assessments of creatine kinase. Thereafter, further electrocardiogram and enzyme evaluations were performed if clinically indicated. A non—Q-wave myocardial infarction was defined as an increase in the creatine kinase level to 3 times the upper limit of the normal range.
Angiographic StudyThe baseline bifurcation anatomy was assessed according to the Medina et al classification.6 Pre- and postpercutaneous coronary intervention angiographies were performed using the CAAS II version 4.1.1 (Pie Medical Imaging; Maastricht, Netherlands) or the CardiOp-B system7 for quantitative coronary angiography. The dye-filled catheter was used as a reference. The following parameters were obtained from the pre- and postpercutaneous coronary intervention angiography: the reference diameter, the minimal lumen diameter, and the percent stenosis of the MV, as well as the reference diameter, minimal lumen diameter, and percent stenosis of the SB. An intravascular ultrasound study was performed at the operator's discretion.
Follow-up StudyAfter discharge, patients were followed-up by hospital visit or by a telephone interview at 1 year, 18 months (SEAside trial patients) and 3 years. Patients with symptom recurrence or with inducible ischemia were recommended to undergo angiographic evaluation. The clinical events were defined as follows: a) death: the cause of death was ascertained by reviewing the available clinical records, and all deaths without a clear noncardiac cause were considered to be cardiac deaths; b) acute myocardial infarction: defined as a typical rise and fall of biochemical markers of myocardial necrosis with ischemic symptoms or electrocardiogram changes, 24h after the index procedure; c) target vessel revascularization: repeat percutaneous coronary intervention or coronary surgery on the target vessel due to recurrent ischemia; d) MACE: death or acute myocardial infarction or target vessel revascularization, and e) stent thrombosis: defined according to the Academic Research Consortium criteria8 as “definite” (angiography- or autopsy-confirmed stent thrombosis) or “probable” (any unexplained death within the first 30 days or any myocardial infarction in the territory of the stent and in the absence of any other obvious cause).
Study EndpointsThe primary objective of the study was to analyze the differences in MACE during the 3-year follow-up between the groups of patients treated with an SES or an EES. Secondary endpoints were the individual components of the primary endpoint (death or acute myocardial infarction or target vessel revascularization). Periprocedural myocardial infarction was excluded from MACE.
Statistical AnalysisDichotomous variables are reported as counts and percentages of the total. Quantitative variables are expressed as means (standar deviation [SD]), Differences between the proportions were analyzed by the χ2 or Fisher's exact test, as appropriate. A Student-Fisher unpaired t test was used to compare continuous variables between the 2 groups of patients. Event-free survival curves were generated using Kaplan-Meier methods. The log rank test was used to determine the statistical significance of the differences in the 3-year cumulative event rate between groups. To compare late adverse events, a subanalysis was performed of events occurring after 1 year.
A value of P < .05 was considered to be statistically significant. All analyses were performed using the SPSS 20.0.0 software.
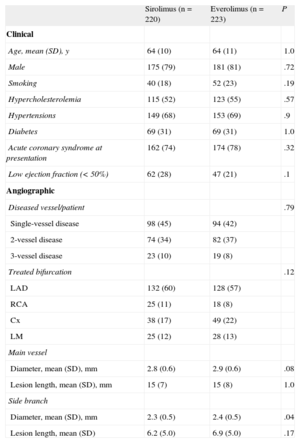
RESULTSBaseline DataBaseline clinical and angiographic data are shown in Table 1. Most of the patients were admitted to hospital due to acute coronary syndrome. Age, sex, risk factors, and clinical characteristics were similar in the 2 groups. A significant proportion of patients were diabetics (31%) with a similar prevalence in the 2 groups. The bifurcation site was most frequently located at the left anterior descending artery/diagonal branch. The 2 groups were similar with respect to bifurcation location, MV size, severity of the stenosis, and the type of bifurcation lesion according to the Medina et al6 classification (Table 2). The mean SB diameter was larger in the EES group than in the SES group (2.3 [SD, 0.5] vs 2.4 [SD, 0.5 mm]; P = .04; Table 1).
Baseline Data
| Sirolimus (n = 220) | Everolimus (n = 223) | P | |
| Clinical | |||
| Age, mean (SD), y | 64 (10) | 64 (11) | 1.0 |
| Male | 175 (79) | 181 (81) | .72 |
| Smoking | 40 (18) | 52 (23) | .19 |
| Hypercholesterolemia | 115 (52) | 123 (55) | .57 |
| Hypertensions | 149 (68) | 153 (69) | .9 |
| Diabetes | 69 (31) | 69 (31) | 1.0 |
| Acute coronary syndrome at presentation | 162 (74) | 174 (78) | .32 |
| Low ejection fraction (< 50%) | 62 (28) | 47 (21) | .1 |
| Angiographic | |||
| Diseased vessel/patient | .79 | ||
| Single-vessel disease | 98 (45) | 94 (42) | |
| 2-vessel disease | 74 (34) | 82 (37) | |
| 3-vessel disease | 23 (10) | 19 (8) | |
| Treated bifurcation | .12 | ||
| LAD | 132 (60) | 128 (57) | |
| RCA | 25 (11) | 18 (8) | |
| Cx | 38 (17) | 49 (22) | |
| LM | 25 (12) | 28 (13) | |
| Main vessel | |||
| Diameter, mean (SD), mm | 2.8 (0.6) | 2.9 (0.6) | .08 |
| Lesion length, mean (SD), mm | 15 (7) | 15 (8) | 1.0 |
| Side branch | |||
| Diameter, mean (SD), mm | 2.3 (0.5) | 2.4 (0.5) | .04 |
| Lesion length, mean (SD) | 6.2 (5.0) | 6.9 (5.0) | .17 |
Cx, circumflex artery; LAD, left anterior descending artery; LM, left main; RCA, right coronary artery; SD: standard deviation.
Unless otherwise indicated, the data are expressed as No. (%).
Type of Bifurcation According to the Medina et al6 Classification
| Sirolimus (n = 220) | Everolimus (n = 223) | P | |
| 1,1,1 | 69 (31) | 96 (43) | .12 |
| 1,1,0 | 66 (30) | 54 (24) | |
| 1,0,1 | 8 (4) | 4 (2) | |
| 0,1,1 | 20 (9) | 16 (7) | |
| 1,0,0 | 24 (11) | 19 (8.5) | |
| 0,1,0 | 29 (13) | 33 (15) | |
| 0,0,1 | 4 (2) | 1 (0.5) |
Data are expressed as no. (%).
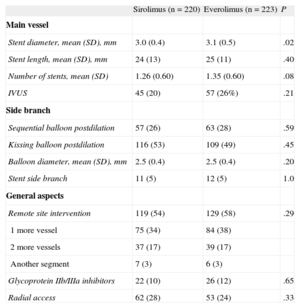
The procedural characteristics are summarized in Table 3. The lesions in the 2 groups were treated similarly. The SB was postdilated in the majority of the patients. The use of a second stent at the SB was low and was almost identical in both groups (5%). There were no significant differences between the groups with regard to other technical aspects or the use of platelet glycoprotein IIb/IIIa inhibitors.
Procedural Data
| Sirolimus (n = 220) | Everolimus (n = 223) | P | |
| Main vessel | |||
| Stent diameter, mean (SD), mm | 3.0 (0.4) | 3.1 (0.5) | .02 |
| Stent length, mean (SD), mm | 24 (13) | 25 (11) | .40 |
| Number of stents, mean (SD) | 1.26 (0.60) | 1.35 (0.60) | .08 |
| IVUS | 45 (20) | 57 (26%) | .21 |
| Side branch | |||
| Sequential balloon postdilation | 57 (26) | 63 (28) | .59 |
| Kissing balloon postdilation | 116 (53) | 109 (49) | .45 |
| Balloon diameter, mean (SD), mm | 2.5 (0.4) | 2.5 (0.4) | .20 |
| Stent side branch | 11 (5) | 12 (5) | 1.0 |
| General aspects | |||
| Remote site intervention | 119 (54) | 129 (58) | .29 |
| 1 more vessel | 75 (34) | 84 (38) | |
| 2 more vessels | 37 (17) | 39 (17) | |
| Another segment | 7 (3) | 6 (3) | |
| Glycoprotein IIb/IIIa inhibitors | 22 (10) | 26 (12) | .65 |
| Radial access | 62 (28) | 53 (24) | .33 |
IVUS, intravascular ultrasound; SD, standard deviation.
Unless otherwise indicated, the data are expressed as No. (%).
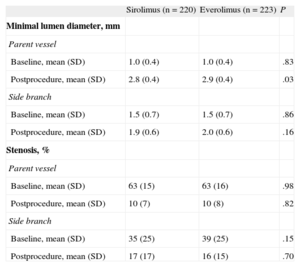
Quantitative angiographic data are summarized in Table 4. The mean minimal luminal diameter of the treated segments and the reduction in stenosis immediately after the procedure were similar in the MV and in the SB in both groups. The in-hospital and 1-year follow-up outcomes have previously been reported.3,4 The rate of major events during the first year of follow-up was low in both groups, with no significant differences. Table 5 summarizes these data.
Quantitative Angiographic Study
| Sirolimus (n = 220) | Everolimus (n = 223) | P | |
| Minimal lumen diameter, mm | |||
| Parent vessel | |||
| Baseline, mean (SD) | 1.0 (0.4) | 1.0 (0.4) | .83 |
| Postprocedure, mean (SD) | 2.8 (0.4) | 2.9 (0.4) | .03 |
| Side branch | |||
| Baseline, mean (SD) | 1.5 (0.7) | 1.5 (0.7) | .86 |
| Postprocedure, mean (SD) | 1.9 (0.6) | 2.0 (0.6) | .16 |
| Stenosis, % | |||
| Parent vessel | |||
| Baseline, mean (SD) | 63 (15) | 63 (16) | .98 |
| Postprocedure, mean (SD) | 10 (7) | 10 (8) | .82 |
| Side branch | |||
| Baseline, mean (SD) | 35 (25) | 39 (25) | .15 |
| Postprocedure, mean (SD) | 17 (17) | 16 (15) | .70 |
SD, standard deviation.
Data are expressed as mean (standard deviation).
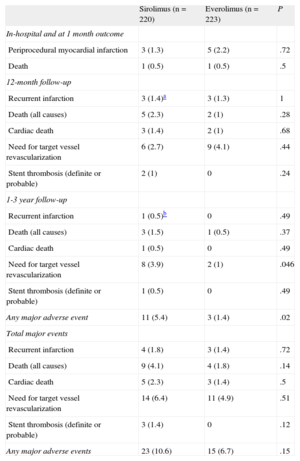
Major Adverse Cardiovascular Events and Follow-up Results
| Sirolimus (n = 220) | Everolimus (n = 223) | P | |
| In-hospital and at 1 month outcome | |||
| Periprocedural myocardial infarction | 3 (1.3) | 5 (2.2) | .72 |
| Death | 1 (0.5) | 1 (0.5) | .5 |
| 12-month follow-up | |||
| Recurrent infarction | 3 (1.4)a | 3 (1.3) | 1 |
| Death (all causes) | 5 (2.3) | 2 (1) | .28 |
| Cardiac death | 3 (1.4) | 2 (1) | .68 |
| Need for target vessel revascularization | 6 (2.7) | 9 (4.1) | .44 |
| Stent thrombosis (definite or probable) | 2 (1) | 0 | .24 |
| 1-3 year follow-up | |||
| Recurrent infarction | 1 (0.5)b | 0 | .49 |
| Death (all causes) | 3 (1.5) | 1 (0.5) | .37 |
| Cardiac death | 1 (0.5) | 0 | .49 |
| Need for target vessel revascularization | 8 (3.9) | 2 (1) | .046 |
| Stent thrombosis (definite or probable) | 1 (0.5) | 0 | .49 |
| Any major adverse event | 11 (5.4) | 3 (1.4) | .02 |
| Total major events | |||
| Recurrent infarction | 4 (1.8) | 3 (1.4) | .72 |
| Death (all causes) | 9 (4.1) | 4 (1.8) | .14 |
| Cardiac death | 5 (2.3) | 3 (1.4) | .5 |
| Need for target vessel revascularization | 14 (6.4) | 11 (4.9) | .51 |
| Stent thrombosis (definite or probable) | 3 (1.4) | 0 | .12 |
| Any major adverse events | 23 (10.6) | 15 (6.7) | .15 |
Data are expressed as No. (%).
Clinical follow-up at 3 years was available in 439 out of 443 patients (99.1%). Event rates for both groups are shown in Table 5. MACE at 3 years occurred in 23 patients (10.6%) in the SES group vs 15 (6.7%) in the EES group (P = .15). The incidences of death, target vessel revascularization, and myocardial infarction were also similar in the 2 groups. Definite or probable stent thrombosis occurred in 3 patients (1.4%) in the SES group and in none in the EES group. Figure 2 shows the probability of survival free of a MACE at 3 years in both groups of patients. Although the overall rate of MACE was not statistically significantly different between the 2 groups, a separation of the curves beyond 1 year was noted. Thus, a subanalysis of events beyond 1 year showed a significant difference in terms of target vessel revascularization and total MACE in favor of the EES group (Figure 3). During this period, there were 4 additional deaths: 1 fatal myocardial infarction in the SES group and 3 noncardiac deaths, 2 pulmonary neoplasms, and 1 aortic aneurysm (Table 5). Figure 3 depicts the Kaplan-Meier curves for survival beyond 1 year free of overall MACE in both groups of patients.
Bifurcation lesions constitute a challenging subset of coronary lesions. DES may be implanted in bifurcated lesions using different techniques, with provisional SB stenting being the currently preferred strategy. Although SES have been found to be the most efficacious stents for the percutaneous treatment of bifurcation lesions during the last decade,9–13 the second generation of DES might theoretically further improve the clinical outcome of these patients.14
Everolimus- vs Sirolimus-eluting Stents in Bifurcation Lesions: the Present StudyIn this study, we performed a pooled analysis of the 2 existing randomized studies that have compared a first generation DES (ie, SES) with a latest-generation DES (ie, EES) in the specific and challenging setting of bifurcation lesions. The study population investigated had overall high-risk clinical and angiographic features as reflected by the 75% rate of acute coronary syndromes and the 70% rate of target bifurcation location in the distal left main stem or left anterior descending artery. Percutaneous coronary intervention was conducted according to the provisional stenting technique, which, to date, represents the gold standard for unselected bifurcation lesions. At 3 years’ follow-up, the overall incidence of MACE was low, with these events occurring in 6.7% of the EES group vs 10.6% of the SES group (P = .15). Because late events may differ between DES and because survival curves showed a separation after 1 year, late events were specifically investigated in the present study. The results of this subanalysis showed that the occurrence of new events stabilized after 1 year in the EES group, with a significantly lower occurrence of target vessel revascularization and MACE compared with SES in the time interval between 1 year and 3 years. Among the possible mechanisms potentially explaining the lower occurrence of late events with EES than with SES, both the suitability of the platform for bifurcation stenting and long-term healing may play a role. Indeed, side cell opening toward the SB has been documented to be better with EES than with SES15 and to result in a better 3-dimensional SB ostium angiographic result.3 These issues may determine a different flow pattern at the bifurcation level, which may influence local shear stress forces and consequently vessel wall response to the stent.16 Regarding the long term healing process, a recent optical coherence tomography study showed that, beyond 1-year follow-up, EES had a lower rate of uncovered and nonappositioned struts compared with SES.17
Previous Studies Comparing Everolimus- vs Sirolimus-eluting Stents in Nonbifurcation LesionsSix meta-analyses comparing the efficacy and safety of EES vs other DES (including SES) have recently been published.18–23 All of them pooled the results of randomized trials without a very long clinical follow-up and reached similar conclusions: compared with other DES, EES seem to be associated with a significant reduction of stent thrombosis, an effect that appears early and increases in magnitude through the follow-up. Mortality, target vessel revascularization and MACE were not significantly improved with EES. Among diabetics, a recent meta-analysis of trials testing 4 DES (including SES and EES), compared with each other or with bare metal stents, suggested that EES may be the most efficacious and safe.22 These observations fit well with the lower rates of target lesion revascularization and MACE observed in our study and suggest that longer follow-up may be needed to detect differences in the clinical performance between the first- and latest-generation DES.
LimitationsInformation about medical treatment after 1-year of follow-up was unavailable. Differences in antithrombotic regimens could determine patient outcome after long-term follow up.
ConclusionsProvisional SB stenting with either SES or EES in bifurcation lesions is associated with low rates of MACE at 3 years’ follow-up. The results of a subanalysis of events after 1 year showing a lower event rate with EES compared with SES suggests that studies exploring the long-term clinical benefit of the latest generation of DES are warranted.
CONFLICTS OF INTERESTManuel Pan: Minor lecture fees from Abbott. Francesco Burzotta: Minor lecture fees from Medtronic.