Each year, more than 200 million patients undergo noncardiac surgery worldwide,1 and 1 million of these patients die within 30 days following surgery. Major vascular complications (vascular cause of death, nonfatal myocardial infarction, nonfatal cardiac arrest, and nonfatal cerebrovascular accident) are the main cause of morbidity and mortality in these patients, and acute myocardial infarction (AMI) is the most frequent among them (5.7%).2
The physiopathology of perioperative myocardial infarction is currently a topic of debate, involving 2 potential mechanisms. The first is the formation of a thrombus in the coronary artery due to the inflammatory state and hypercoagulability induced by surgical stress and tissue injury.3,4 Recent studies have shown that patients with perioperative acute coronary syndrome have angiographic findings compatible with thrombotic complications, and the frequency of these findings is similar to that of patients with acute coronary syndrome outside the surgical context.5 The second mechanism is the imbalance between the myocardial supply and oxygen demand.6 On the one hand, the physiological response to surgical stress, which persists for several days after the intervention, increases oxygen consumption; on the other, several common circumstances during surgery and the postoperative period, such as hypotension, anemia, hypoxia or hypovolemia, reduce oxygen supply.
The American and European cardiology societies have recently updated the universal definition and diagnostic criteria of AMI.7 Acute myocardial infarction is defined as the presence of myocardial necrosis in a clinical context of acute myocardial ischemia, whose diagnosis requires elevation and/or reduction of cardiac biomarker levels (preferably troponin) together with symptoms of ischemia and/or compatible electrocardiographic or echocardiographic findings. Nonetheless, there is growing evidence that the myocardial lesions detected by troponin elevation during the immediate postoperative period (48 h to 72 h after surgery) do not meet these criteria.2 In the immediate postoperative period, patients receive analgesia and some remain sedated and/or on mechanical ventilation, so the majority (65.3%) report no symptoms of ischemia.8 Likewise, electrocardiographic changes are usually transitory and not very expressive, so they frequently go unnoticed.6 Nevertheless, whether or not there are symptoms, the prognosis of perioperative AMI (either type 1 or type 2 according to the universal definition) is poor, with a 30-day mortality rate after surgery of over 11%.8
The estimation of cardiovascular risk in the preoperative period can improve the prognosis of patients with a greater probability of developing cardiovascular complications in the postoperative period. However, the risk prediction models most commonly used to date 9 have limitations and usually underestimate this risk.3,10
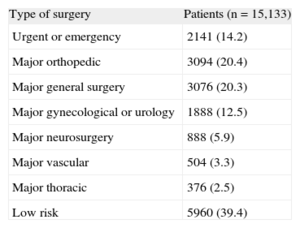
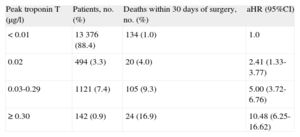
The VISION11 cohort, an international prospective study that evaluated major vascular complications in patients who had undergone noncardiac surgery, observed a postsurgical 30-day mortality rate that was 3 times higher than expected (1.9%). The study included patients older than 45 years who had undergone either elective or urgent surgery with general or regional anesthesia and had stayed at least 1 night in the hospital. Table 1 lists the types of surgery performed in these patients. The first analysis of this cohort included 15 133 patients with troponin T (TnT) determinations 6 h to 12 h after surgery as well as on the first, second and third days after the intervention. The multivariate analysis showed that peak TnT levels ≥ 0.02 ng/mL were powerful independent predictors for 30-day all-cause mortality (Table 2). Therefore, even TnT values considered normal (< 0.04 ng/mL) have important prognostic repercussions.
Types of Surgical Interventions of the Patients Included in the VISION11 Study
| Type of surgery | Patients (n=15,133) |
| Urgent or emergency | 2141 (14.2) |
| Major orthopedic | 3094 (20.4) |
| Major general surgery | 3076 (20.3) |
| Major gynecological or urology | 1888 (12.5) |
| Major neurosurgery | 888 (5.9) |
| Major vascular | 504 (3.3) |
| Major thoracic | 376 (2.5) |
| Low risk | 5960 (39.4) |
Data are expressed as No (%).
Peak Thresholds of Troponin T During the First 3 days of the Postoperative Period That Were Independent Predictors for 30-day Mortality
| Peak troponin T (μg/l) | Patients, no. (%) | Deaths within 30 days of surgery, no. (%) | aHR (95%CI) |
| < 0.01 | 13 376 (88.4) | 134 (1.0) | 1.0 |
| 0.02 | 494 (3.3) | 20 (4.0) | 2.41 (1.33-3.77) |
| 0.03-0.29 | 1121 (7.4) | 105 (9.3) | 5.00 (3.72-6.76) |
| ≥ 0.30 | 142 (0.9) | 24 (16.9) | 10.48 (6.25-16.62) |
95%CI: 95% confidence interval; aHR: adjusted hazard ratio.
Given these results, researchers of the VISION study have recently proposed a new concept called MINS (myocardial injury after noncardiac surgery), defined as all troponin elevations considered ischemic in origin, with prognostic relevance and occurring either during surgery or in the following 30 days.12 After adjusting the regression model not only for preoperative variables but also for perioperative complications, the authors confirmed the independent association of TnT values ≥ 0.03 ng/mL with 30-day all-cause mortality after surgery. The diagnostic criteria for MINS proposed by these researches include a TnT value ≥ 0.03 ng/mL that is ischemic in etiology and occurs in the first 30 days after noncardiac surgery.
The incidence of MINS in the VISION cohort was 8%, and 87.1% of MINS occurred in the first 2 days after surgery. Patients with MINS were older, had more cardiovascular risk factors and known cardiovascular disease, and 84.2% had no symptoms of ischemia. Electrocardiographic changes were seen in 34.9%, the most common of which were T-wave inversion (23.3%) and ST depression (16.4%). Twelve independent predictors for myocardial injury were identified, among them age ≥ 75 years, cardiovascular risk factors (eg, renal failure and diabetes mellitus), known cardiovascular disease, and urgent surgery. Patients with MINS had a greater risk for vascular complications and their mortality rate was higher than that in patients without MINS (9.8 vs 1.1%). Postsurgical 30-day mortality was 13.5% among those with MINS and signs and/or symptoms of ischemia (41.8% of the patients with MINS) and was 7.7% among those with no signs or symptoms of ischemia. A total of 58.2% of the myocardial ischemia complications with prognostic relevance would have gone unnoticed without TnT determination.
Given the poor prognosis of perioperative AMI and its difficult clinical diagnosis,8,12 the consensus document about the third universal definition of AMI7 recommends systematic determination of cardiac biomarkers in the perioperative period (before surgery and 48 h to 72 h afterwards) in patients with high cardiovascular risk who have undergone major surgery. This determination would detect AMI in the absence of signs or symptoms of ischemia, as well as identify patients with myocardial lesions not meeting the proposed criteria but with a high risk of death within 30 days of surgery. The objective is to identify patients at high risk for cardiovascular complications and to offer them an appropriate level of postoperative care.
Among the strategies proposed to reduce the risk of perioperative myocardial injury, currently the most controversial is the use of beta-blockers. For more than a decade, the clinical practice guidelines of the European and American societies for the evaluation of preoperative risk and perioperative cardiovascular management have created recommendations in favor of the use of beta-blockers in patients with vascular risk who undergo intermediate- or high-risk cardiac surgery. Nonetheless, after the publication of the POISE (PeriOperative ISchemic Evaluation) study and the irregularities detected in the DECREASE (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography), studies, the risk/benefit ratio of beta-blockers is currently unfavorable. Of the 2 meta-analyses13,14 published to date that include the POISE study, the most recent excludes the results of some of the DECREASE studies and shows that not only do beta-blockers not reduce the risk for any-cause death within 30 days of surgery, but actually increase it by 27%. In response to this finding, the European and American cardiology societies have announced that they are working on new updates for their clinical practice guidelines. In the interim, their recommendation is that beta-blockers in patients scheduled for noncardiac surgery should not be prescribed systematically; instead, they should be prescribed after careful assessment of their potential risks and benefits. Among the studies included, the POISE study recruited the largest number of patients (n=8351) and was one of the studies with the fewest biases, and therefore its results largely influenced the final results of these meta-analyses. It has been discussed whether the beta-blocker doses used in the POISE study (100 mg of metoprolol succinate 2h to 4h preoperatively and 200mg/day for 30 days postoperatively) could be excessive. Furthermore, the lack of titration according to their effect could have caused the adverse effects observed (a higher number of deaths and strokes), which were attributed to the higher frequency of clinically significant hypotension and bradycardia in the metoprolol group. Nevertheless, other studies with different beta-blockers, doses and routes of administration have also shown a greater frequency of hypotension in the beta-blocker arm.13
It is currently unknown whether there is a particular patient subgroup that could benefit from beta-blockers in the perioperative period of noncardiac surgery; if so, the dose, type of beta-blocker, and safest routes of administration would need to be determined. However, the updates of the European guidelines in 2009,15 based on the POISE study, recommended not administering high beta-blockers doses without prior titration. The feasibility and effectiveness of this preoperative titration, which aims to avoid excessive hypotension and bradycardia, are arguable. On the one hand, preoperative titration, with the current volume of surgery, would cause an unmanageable workload in many centers. On the other, the preoperative dose stipulated is not in any way a guarantee during the procedure or postoperative period, when hypotension is a frequent problem (9.7% of the patients of the placebo group in the POISE study).
Randomized, controlled trials are still necessary to identify interventions that are able to reduce the risk of myocardial injury after noncardiac surgery. Meanwhile, it is more reasonable to evaluate patient risk in order to adjust intraoperative and postoperative monitoring and care accordingly, while avoiding and correcting anemia, tachycardia, and hypotension both during and after the intervention. Surgical techniques that are less aggressive than open surgery can be an alternative to reduce the mortality of older patients with multiple comorbidities and high cardiovascular risk.16
As for the treatment of myocardial injuries after noncardiac surgery, there is currently insufficient evidence regarding which therapies could be effective to improve the 30-day postsurgical prognosis. As with beta-blockers, the measures that have been shown to be beneficial for patients with AMI in the nonsurgical context may not offer the same favorable risk-benefit balance for perioperative AMI. Currently, the international multicenter study Management of Myocardial Injury After Noncardiac Surgery (ClinicalTrials.gov: NCT01661101) is evaluating the effectiveness and safety of oral anticoagulants to reduce 30-day mortality in patients who have had postoperative MINS.
Given these data, the teams involved in the perioperative management of these patients (including cardiology) should be organized to identify at-risk patients and support the diagnosis, risk stratification, treatment, and follow-up of those with myocardial lesions after noncardiac surgery. To this end, the perioperative determination of cardiac biomarkers is recommended. The increased life expectancy and/or quality of life that patients expect from surgery should not be truncated by cardiovascular complications that we have not been able to identify.
CONFLICTS OF INTERESTThe authors of this article are researchers of the VISION study in Spain and have received funding from the study to attend work meetings.