The transcription factor TBX1 plays an important role in the embryonic development of the heart. Nothing is known about its involvement in myocardial remodeling after acute myocardial infarction (AMI) and whether its expression can be modulated by a treatment with proven benefit such as mineralocorticoid receptor blockade.
MethodsAcute myocardial infarction was induced in 60 rats via left coronary artery ligation: 50 animals were randomized to be euthanized after 1, 2, 4, 12, or 24 weeks; 10 animals were treated with eplerenone (100 mg/kg/days) 7 days before the AMI until their euthanasia (4 weeks later); 8 additional animals underwent surgery without ligation (control). We analyzed the cardiac expression of TBX1, fetal genes, and fibrosis markers.
ResultsThe gene and protein expression of TBX1 was increased in the infarcted myocardium, peaking 1 week after AMI (P < .01), without changes in the noninfarcted myocardium. Levels of the fetal genes and fibrosis markers also increased, peaking 4 weeks (P < .001) and 1 week (P < .01) after AMI, respectively. The TBX1 expression was correlated with that of the fibrosis markers (P < .01) but not the fetal genes. Eplerenone reduced the TBX1 increase and fibrosis induced by AMI, with an association improvement in ventricular function and remodeling in echocardiography.
ConclusionsThese results show the reactivated expression of TBX1 and indicate its involvement in cardiac fibrosis and remodeling after AMI and its participation in the benefit from mineralocorticoid receptor blockade.
Keywords
The T-box 1 (TBX1) protein belongs to a family of transcription factors whose members share a DNA binding domain known as the T-box.1 The principal function of TBX1 is to regulate cell proliferation and differentiation during the organogenesis of various tissues, including the heart. In humans, it activates the proliferation of mesenchymal precursor cells contributing to myocardial outflow tract formation and cardiac tube elongation at the anterior pole.2,3 Experimental studies in rats have shown an association between Tbx1 gene depletion and increased susceptibility to congenital heart disease.4 Despite its important role in embryonic development, no function has been described for TBX1 in the adult heart.
During embryonic development, the heart is exposed to an oxygen-poor environment that makes anaerobic metabolism and carbohydrates the main source of energy. After birth, fatty acid oxidation predominates, due to a change in the expression of the genes controlling energy metabolism.5 During acute myocardial infarction (AMI), the heart is exposed to hypoxia and metabolic and hemodynamic stress. The heart responds with reactivation of the fetal gene program and a consequent induction of the expression of the isoforms controlling anaerobic metabolism. A further response is hypertrophy and cardiac remodeling, whose objective is to maintain the contractile capacity of the infarcted heart. After the infarction, the adverse tissue remodeling is morphologically manifested by progressive dilatation of the left ventricle and reduced left ventricular ejection fraction (LVEF), factors associated with worse clinical prognosis. Given the similarities between the embryonic and postinfarction metabolic states, TBX1 could play a role in the myocardial response to infarction and participate in tissue repair, remodeling, and loss of contractility, with consequent therapeutic implications.
Over the years, various drugs have shown a clinical benefit in infarcted patients. In particular, mineralocorticoid receptor antagonists have been associated with not only lower mortality, but also less fibrosis and adverse ventricular remodeling.6,7 The possible pharmacological modulation of TBX1 is of interest because it would support the role of TBX1 in post-AMI remodeling and its use as a therapeutic target.
The present study evaluated the expression of the fetal gene Tbx1 in adult hearts subjected to AMI and the relationship of Tbx1 with fibrosis, a process involved in cardiac remodeling after AMI, as well as its possible modulation by eplerenone, a drug with proven clinical benefit after infarction and associated with reduced myocardial fibrosis.8
METHODSThe study was approved by the Ethics Committee of the University of Murcia (number A13150105). A detailed description of the materials and methods can be found in the supplementary material.
Experimental DesignWister rats were used for the AMI model. Of 60 animals that underwent permanent ligation of the left anterior descending coronary artery, 50 (10 rats per group) were randomly assigned to the following groups according to the date of euthanasia: 1, 2, 4, 12, or 24 weeks after AMI. Ten animals were orally administered (mixed in food) an aldosterone antagonist (eplerenone, 100 mg/kg/day). The eplerenone treatment was begun 7 days before the AMI to ensure its effect in the period immediately after the AMI and was maintained until the euthanasia of the animals (4 weeks after AMI). The dose was adjusted on a weekly basis according to the weight of each animal. In addition, 8 animals underwent the same experimental protocol as those euthanized at 4 weeks after AMI but without coronary ligation (control group). The mortality rate in the 24 hours after infarction induction was 29% in untreated animals and 25% in eplerenone-pretreated rats. There was no death in the control group and no animal died more than 24 hours after AMI. In all animals, adverse remodeling was evaluated in terms of dimensions and contractility via a complete echocardiographic study at baseline, 24 hours before surgery, and the day before euthanasia, as described in the supplementary material. This examination was performed by a qualified and experienced operator (M.J. Fernández-Del Palacio) who was blinded to the eplerenone treatment group. At euthanasia, the infarcted and noninfarcted regions of the left ventricular myocardium were separated and stored at –80°C for subsequent analysis. A fragment of the infarcted myocardium was fixed in 10% buffered formalin and embedded in paraffin for subsequent histological analysis.
Ribonucleic Acid Extraction and Quantitative Real-time Polymerase Chain ReactionCommercial kits (supplementary material) were used for the isolation of RNA, its retrotranscription to complementary DNA, and real-time polymerase chain reaction. The primers are described in Table S1 of the supplementary material.
Western Blot AnalysisThe protein expression of TBX1 was evaluated using Western blot: 40 μg protein lysates were separated using SDS-PAGE and transferred to PVDF membranes. The primary antibody was anti-TBX1 (ab109313, Abcam; Cambridge, Massachusetts, United States) and the secondary antibody was horseradish peroxidase-conjugated antirabbit immunoglobulin G (W4011; Promega, Madison, Wisconsin, United States). The TBX1 expression results were normalized to those of the total protein loaded and are expressed relative to those of the control group.
Histological AnalysisFour 5-μm slices were obtained from each paraffin block. These sections were stained with Masson trichrome according to the standard protocol. High-resolution images of the stained sections were obtained with a Leica SN400F slide scanner (Leica Microsystems Inc.; Buffalo Grove, Illinois, United States). The collagen volume fraction was calculated with the Leica Qwin Pro V3.4.0 program (Leica Microsystems, Ltd.; Switzerland) in 40 × magnified images as the average of all of the slices and expressed as the ratio between the area of the collagen stained with Masson trichrome and the total area of the myocardium.
Statistical AnalysisData are expressed as the mean ± standard error or mean ± standard deviation and were statistically analyzed using SPSS 19 (SPSS, Inc.; United States). A Student t test or Mann-Whitney U test was used according to the type of variable. Correlation analysis was performed by excluding the control group animals. Graphs were plotted using SigmaPlot 11.0 software. P values <.05 were considered to indicate statistical significance.
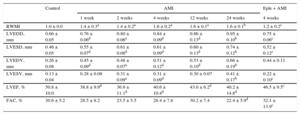
RESULTSEchocardiographic Analysis of the Changes After Acute Myocardial InfarctionThe echocardiographic analysis results are shown in Table 1. From week 1 after AMI, the animals showed decreased systolic function and increased adverse structural remodeling over time, with lower LVEF values and an increased wall motion score index, as well as greater end-diastolic and end-systolic volumes and diameters.
Descriptive Analysis of the Echocardiographic Characteristics of the Different Animal Groups
| Control | AMI | Eple + AMI | |||||
|---|---|---|---|---|---|---|---|
| 1 week | 2 weeks | 4 weeks | 12 weeks | 24 weeks | 4 weeks | ||
| RWMI | 1.0 ± 0.0 | 1.4 ± 0.3a | 1.4 ± 0.2a | 1.6 ± 0.2a | 1.6 ± 0.1a | 1.6 ± 0.1b | 1.2 ± 0.2c |
| LVEDD, mm | 0.66 ± 0.05 | 0.76 ± 0.06d | 0.80 ± 0.06a | 0.84 ± 0.09d | 0.86 ± 0.13d | 0.95 ± 0.10b | 0.75 ± 0.06c |
| LVESD, mm | 0.46 ± 0.05 | 0.55 ± 0.07d | 0.61 ± 0.08d | 0.61 ± 0.09d | 0.60 ± 0.13d | 0.74 ± 0.12b | 0.52 ± 0.12c |
| LVEDV, mm | 0.26 ± 0.08 | 0.45 ± 0.09d | 0.48 ± 0.07a | 0.51 ± 0.12d | 0.53 ± 0.10d | 0.66 ± 0.19b | 0.44 ± 0.11 |
| LVESV, mm | 0.13 ± 0.04 | 0.28 ± 0.08 | 0.31 ± 0.09a | 0.31 ± 0.09d | 0.30 ± 0.07 | 0.41 ± 0.17b | 0.22 ± 0.10c |
| LVEF, % | 50.8 ± 10.0 | 38.8 ± 9.9d | 36.9 ± 11.1d | 40.6 ± 10.4d | 43.0 ± 6.2d | 40.2 ± 14.8d | 46.5 ± 9.5c |
| FAC, % | 30.6 ± 5.2 | 28.5 ± 8.2 | 23.5 ± 5.5 | 28.4 ± 7.8 | 30.2 ± 7.4 | 22.4 ± 5.9d | 32.1 ± 13.9c |
AMI, acute myocardial infarction; Eple, eplerenone; FAC, fractional area change; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic diameter; RWMI: regional wall motion index.
Values are expressed as mean ± standard deviation.
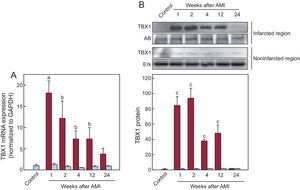
Measurement of Tbx1 expression in cardiac tissue (Figure 1) using quantitative real-time polymerase chain reaction and Western blot showed that the concentration of TBX1 mRNA in the infarcted region was significantly increased from the first week after AMI and was persistently higher than that of the control group until 24 weeks after AMI (Figure 1A). The expression peaked 1 week after AMI. The increased gene expression of Tbx1 corresponded with a higher concentration of TBX1 protein in the infarcted region than in the control group (Figure 1B). Analysis of TBX1 gene and protein expression in the noninfarcted region failed to show significant changes in any of the infarcted animal groups compared with the control group.
Cardiac expression of TBX1 after AMI. A: expression of TBX1 mRNA in the infarcted (red bars) and noninfarcted (blue bars) myocardium; data normalized with respect to GAPDH and compared with the control. B: representative images of Western blotting and densitometric analysis of the expression of TBX1 protein in the infarcted (red bars) and noninfarcted (blue bars) myocardium; data compared with control and normalized with respect to the total protein loaded: staining with AB for the infarcted region and Erk for the noninfarcted region. AB, amido black; AMI, acute myocardial infarction; Erk, extracellular signal-regulated kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger ribonucleic acid; TBX1, T-box 1 protein. aP < .01 vs the control group. bP < .05 vs the control group. cP < .001 vs the control group.
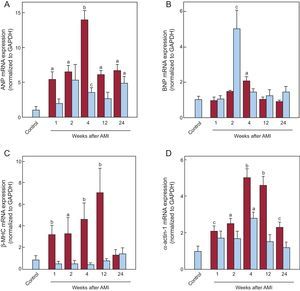
The mRNA expression of the fetal genes in the infarcted and noninfarcted myocardium is shown in Figure 2. The genes examined were atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), beta-myosin heavy chain isoform (β-MHC), and α-actin 1. The kinetics of ANP, β-MHC, and alpha-actin 1 in the infarcted myocardium were similar, with significant increases from the first week after AMI and peak expression at 4 and 12 weeks after AMI. The expression of BNP was only significantly higher than in the control group 4 weeks after AMI. In the noninfarcted myocardium, there was a significant increase in ANP and alpha-actin 1 at 4 weeks after AMI, whereas BNP was increased at 2 weeks. There was no increase in the expression of β-MHC mRNA. Correlation analysis failed to show any association among the expression levels of TBX1 and the other fetal genes analyzed in either the infarcted (Table 2) or noninfarcted (Table S2 of the supplementary material) myocardium. However, the expression of ANP, BNP, β-MHC, and alpha-actin 1 were significantly correlated with each other in the infarcted myocardium (ANP vs BNP, r = 0.642; P < .001; ANP vs alpha-actin 1, r = 0.634; P < .001; BNP vs alpha-actin 1, r = 0.392; P = .012; β-MHC vs alpha-actin 1, r = 0.483; P = .001), with some correlation in the noninfarcted myocardium (ANP vs BNP, r = 0.49; P = .002; ANP vs β-MHC, r = 0.333; P = .04).
Cardiac expression of fetal genes after AMI. mRNA expression of ANP (A), BNP (B), β-MHC (C), and α-actin 1 (D) in the infarcted (red bars) and noninfarcted (blue bars) myocardium. Data normalized with respect to GAPDH and compared with control. AMI, acute myocardial infarction; ANP, atrial natriuretic peptide; β-MHC, beta-myosin heavy chain; BNP, brain natriuretic peptide; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger ribonucleic acid. aP < .01 vs the control group. bP < .001 vs the control group. cP < .05 vs the control group.
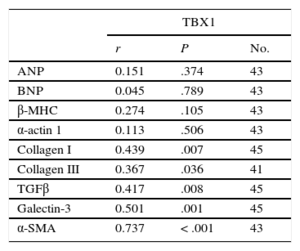
Correlation Analysis Between TBX1 mRNA Expression and the Expression of the Fetal Genes and Fibrosis Markers in the Infarcted Myocardium
| TBX1 | |||
|---|---|---|---|
| r | P | No. | |
| ANP | 0.151 | .374 | 43 |
| BNP | 0.045 | .789 | 43 |
| β-MHC | 0.274 | .105 | 43 |
| α-actin 1 | 0.113 | .506 | 43 |
| Collagen I | 0.439 | .007 | 45 |
| Collagen III | 0.367 | .036 | 41 |
| TGFβ | 0.417 | .008 | 45 |
| Galectin-3 | 0.501 | .001 | 45 |
| α-SMA | 0.737 | < .001 | 43 |
α-SMA, alpha-smooth muscle actin; ANP, atrial natriuretic peptide; β-MHC, beta-myosin heavy chain; BNP, brain natriuretic peptide; TBX1, T-box 1 protein; TGFβ, transforming growth factor beta.
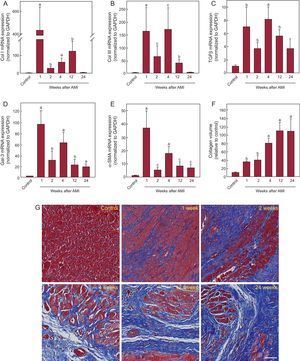
The fibrosis markers analyzed had significantly higher expression in the AMI group than in the control group (Figure 3). All markers showed significantly increased expression from week 1 after AMI, with peaks between weeks 1 and 4 and a progressive reduction at weeks 12 and 24. The increased gene expression of the fibrosis markers led to a progressive increase in interstitial fibrosis in the infarcted myocardium, as shown by histological analysis (Figure 3F). Collagen deposition was evident from the first week after AMI and continued until it completely replaced the cardiac muscle. Analysis revealed a significant correlation between the expression of TBX1 in the infarcted myocardium and that of each of the fibrosis markers analyzed (Table 2). The fibrosis markers were significantly correlated among each other (Table S3 of the supplementary material).
Analysis of cardiac fibrosis after AMI. mRNA expression of collagen I (A), collagen III (B), TGFβ (C), Gal-3 (D), and α-SMA (E). Data normalized with respect to GAPDH and compared with control. Using Masson trichrome staining, the collagen volume was determined in the infarcted myocardium (F). Representative photomicrographs (40×) and quantitative analysis of interstitial fibrosis expressed as collagen volume fraction (G). α-SMA, alpha-smooth muscle actin; AMI, acute myocardial infarction; Col I, collagen I; Col III, collagen III; Gal-3, galectin-3; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger ribonucleic acid; TGFβ, transforming growth factor beta. aP < .001 vs the control group. bP < .01 vs the control group. cP < .05 vs the control group.
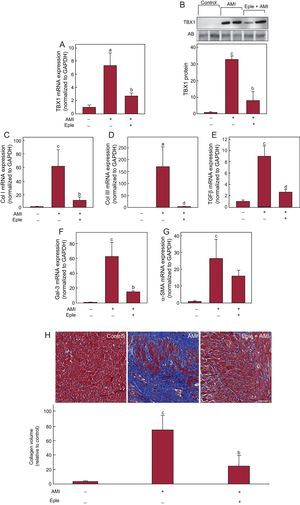
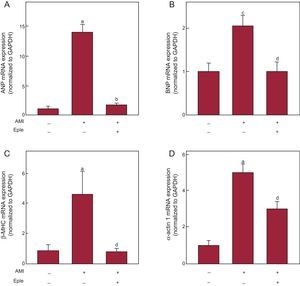
Echocardiographic analysis showed less adverse remodeling after AMI in eplerenone-treated infarcted animals than in untreated infarcted animals, without reaching the levels of the control group (Table 1). Eplerenone administration significantly blocked the increase in TBX1 expression induced by AMI (Figure 4), both mRNA (Figure 4A) and protein (Figure 4B). Eplerenone treatment reduced the fibrosis marker expression in the infarcted myocardium compared with the untreated infarcted animals (Figures 4C-G). This reduced fibrosis marker expression was reflected in a significantly lower collagen deposition in the eplerenone-treated animals than in untreated animals (Figure 4H). Similarly, eplerenone treatment blocked the infarction-induced increase in the expression of the fetal genes (Figure 5).
Effect of eplerenone on the expression of TBX1 and cardiac fibrosis induced after AMI. A: mRNA expression of TBX1 in the infarcted myocardium of rats 4 weeks after AMI induction, with or without previous eplerenone treatment. B: representative images of Western blotting and densitometric analysis of the expression of TBX1 protein in the infarcted myocardium. C-G: mRNA expression analysis of collagen I, collagen III, TGFβ, Gal-3, and α-SMA. H: representative photomicrographs (40×) and quantitative analysis of interstitial fibrosis expressed as collagen volume fraction. α-SMA, alpha-smooth muscle actin; AB, amido black; AMI, acute myocardial infarction; Col I: collagen I; Col III: collagen III; Eple, eplerenone; Gal-3, galectin-3; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger ribonucleic acid; TBX1, T-box 1 protein; TGFβ, transforming growth factor beta. RNA expression data normalized with respect to GAPDH; protein expression normalized with respect to the total protein loaded measured using AB staining. Data compared with control. aP < .05 vs the control group. bP < .05 vs the AMI group. cP < .001 vs the control group. dP < .01 vs the AMI group.
Effect of eplerenone on the expression of fetal genes after AMI. mRNA expression of ANP (A), BNP (B), β-MHC (C), and α-actin 1 (D) in the infarcted myocardium of rats 4 weeks after AMI induction, with or without previous eplerenone treatment. AMI, acute myocardial infarction; ANP, atrial natriuretic peptide; β-MHC, beta-myosin heavy chain; BNP, brain natriuretic peptide; Eple, eplerenone; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger ribonucleic acid. Data normalized with respect to GAPDH and compared with control. aP < .001 vs the control group. bP < .001 vs the AMI group. cP < .01 vs the control group. dP < .01 vs the AMI group.
The present study shows that cardiac expression of TBX1, a transcription factor involved in the embryonic development of the heart, is reactivated in response to a stress situation such as AMI. This reactivation appears to be associated with its participation in cardiac remodeling, because its expression in infarcted tissue was positively correlated with that of fibrosis markers in the weeks after the infarction. The results also indicate some value of TBX1 as a therapeutic target, given that eplerenone treatment was associated with lower expression of both AMI-associated TBX1 and fibrosis. As far as we know, this study is the first to link the TBX1 transcription factor with a function in the adult heart and the pathophysiology of AMI.
TBX1 and Reactivation of Fetal Genes After Acute Myocardial InfarctionAfter an AMI, the heart suffers a series of cellular and molecular events collectively known as cardiac remodeling, with the initial compensatory effects eventually leading to a failing heart. A distinctive signature of post-AMI remodeling is the reactivation of the fetal gene program due to an altered neurohormonal response and the hemodynamic and metabolic stress dealt to the infarcted heart.9 The characteristic changes include remodeling of the cardiomyocyte contractile machinery, with increased expression of sarcomeric proteins (heavy chain myosins and alpha-actin), changes in energy metabolism, and increased production of natriuretic peptides (ANP and BNP).10,11 The initial objective of these changes is to maintain the contractile capacity and efficiency of the cardiac tissue, but their prolongation leads to pathological remodeling with ventricular dilatation and heart failure.
As shown in the present study, the expression of fetal genes is reactivated after AMI, as shown by increased mRNA levels of β-MHC, alpha-actin 1, ANP, and BNP in the infarcted myocardium. This altered expression was less evident in the noninfarcted myocardium. Various transcription factors have been implicated in the reactivation of these fetal genes in a stressed adult heart, such as Nkx2.5, MEF2, GATA4, and SRF.5,12,13 Although these transcription factors participate in the embryonic development of the heart, their expression is reactivated in the infarcted heart to trigger the structural remodeling of the myocardium. Our results are the first to show increased expression of the TBX1 transcription factor in the adult heart from the initial post-AMI phase. This increase in TBX1 was not correlated with the expression of any of the known fetal genes evaluated, indicating that TBX1 has no direct involvement in the expression of the genes controlling sarcomerogenesis (β-MHC and alpha-actin 1) and the natriuretic peptides (ANP and BNP).
TBX1 and its Relationship With Fibrosis Induced by Acute Myocardial InfarctionCardiac remodeling after AMI is mediated by a large number of cytokines released by cardiomyocytes, fibroblasts, endothelial cells, and inflammatory cells.14 Transforming growth factor beta (TGFβ) and the lectin galectin-3 are 2 indispensable molecules for the induction of cardiac fibrosis after AMI.15,16 Both can bind to receptors present in cardiac fibroblasts and can convert interstitial fibroblasts into myofibroblasts through p-Smad2/3-mediated signaling. These cells control the synthesis and extracellular deposition of collagen, which promotes myocardial scar formation and AMI extension. Our results showed that expression of collagen I and III mRNA increased from the first post-AMI week and that the volume of their deposition rises progressively, facilitating scar formation. As expected, the increased levels of collagen I and III mRNA were correlated with the expression of TGFβ and galectin-3, molecules involved in fibroblast activation, and with the expression of alpha-smooth muscle actin, a molecular marker of activated myofibroblasts (Tables S3 of the supplementary material). It was thus interesting that TBX1 expression was positively correlated with the expression of collagen and the molecules controlling the activation of the fibrotic response. This positive correlation indicated a modulatory effect of the TBX1 transcription factor on the genes involved in fibrosis activation after AMI. This hypothesis was strengthened by a recent study showing that rats subjected to acute renal damage induced by gentamicin had increased levels of TBX1 and TGFβ-activated Smad2/3 signaling.17 In contrast, NRK renal cells deficient in TBX1 exhibited lower levels of TGFβ and reduced activation of Smad2/3 signaling. After AMI, TGFβ/Smad signaling plays a major role in fibrosis induction.18 In accordance with these findings, our results also indicate that increased TBX1 in the infarcted region could play a role in the activation of cardiac fibroblasts and the transcriptional activation of the genes controlling cardiac fibrosis after AMI.
TBX1: Potential Therapeutic Target in Acute Myocardial InfarctionRemodeling after AMI significantly contributes to heart failure and death through left ventricular dilatation and systolic dysfunction and ventricular arrhythmogenesis. Cardiac fibrosis is recognized as an important part of the pathogenesis of this unfavorable remodeling and its inhibition improves cardiac function.19 Mineralocorticoid receptor blockade (via spironolactone and eplerenone) improves the prognosis of patients with systolic heart failure.6,20,21 In patients with AMI and LVEF < 35%, eplerenone administration improves survival and decreases sudden cardiac death and progression to heart failure.6,22 Accordingly, the benefits of these drugs have been linked to their ability to reduce myocardial fibrosis, via inhibition of the aldosterone-induced collagen increase.7,8,23 Numerous experimental studies have shown the long-term benefit of antifibrotic therapies, due to reduced dilatation and systolic dysfunction.7,24,25 In our study, eplerenone treatment attenuated the TBX1 increase induced by AMI, decreased the fibrosis marker expression, and led to echocardiography-determined recovery of cardiac function. The molecular mechanisms explaining the antifibrotic effect of the aldosterone antagonists are poorly understood. Their effects may be mediated by different intracellular response systems, such as signaling via galectin-3, the IL-33/ST2 system, and TGF/Smad and the inflammatory response. There is currently no evidence connecting TBX1 with the mineralocorticoid receptor. However, our study indicates that inhibition of the expression of the TBX1 transcription factor is involved in the antifibrotic effect of eplerenone in AMI treatment. Thus, TBX1 could be a therapeutic target to block adverse cardiac remodeling after AMI.
LimitationsThe observation of the mediating role of the TBX1 transcription factor in fibrosis after AMI was based on results obtained from a correlation analysis of the mRNA expression levels, without evaluation of the association of protein expression. To confirm the involvement of TBX1 in post-AMI fibrosis and rule out its participation in the reactivation of the fetal genes controlling the synthesis of sarcomeric proteins and natriuretic peptides, future experimental studies should evaluate the effects of TBX1 modulation (via silencing or overexpression of the Tbx1 gene) on the fibrosis and remodeling process. The dosage of eplerenone (100 mg/kg/day), standard for experimental studies of animals, is higher than that used in clinical practice, possibly representing a limitation when translating the results to the clinical situation.
CONCLUSIONSThe present study characterized the spatiotemporal modulation of TBX1 in heart tissue after AMI. The expression of TBX1 is increased in the infarcted myocardium from the initial phase after AMI and remains elevated. This modulation is correlated with the activation kinetics of the genes of the different fibrosis markers analyzed, identifying the TBX1 transcription factor as a new potential therapeutic target to protect the infarcted heart and reduce fibrosis and adverse remodeling. The aldosterone antagonist eplerenone blocks TBX1 expression reactivation while reducing the profibrotic response, which is associated with better cardiac function. The reactivation of TBX1 in infarction and its regulation by eplerenone indicates the need for new studies aimed at identifying the mechanism of TBX1 reactivation after infarction and its importance as a therapeutic target in the remodeling processes.
FUNDINGThis work has been funded by the Fundación Séneca-Agencia de Ciencia y Tecnología of the Region of Murcia (19334/PI/14), the Instituto de Salud Carlos III, Madrid (PI14/01637), and the Red de Investigación Cardiovascular (RIC), Ministerio de Sanidad y Consumo, Madrid, Spain (RD12/0042/0049).
CONFLICTS OF INTERESTNone declared.
- –
The transcription factor TBX1 participates in embryonic heart development but is no longer expressed in the adult heart. Although no function for TBX1 has been found in the adult heart, a fibrotic role for TBX1 was recently found in animal models of renal damage. As with other fetal genes, TBX1 expression could be reactivated after a myocardial infarction, allowing it to play a role in the pathophysiology of the infarction.
- –
The present study is the first to evaluate TBX1 modulation in the hearts of adult animals subjected to myocardial infarction. After infarction, TBX1 expression was reactivated in the infarcted region, which seemed to be associated with the fibrotic response after infarction. Treatment with the aldosterone antagonist eplerenone blocked the reactivation of TBX1 expression and thereby decreased the profibrotic response and improved cardiac function. These results suggest that TBX1 could be a new therapeutic target to protect the infarcted heart.