Little is known about the risks and outcomes of pregnancy in women with Brugada syndrome. We therefore evaluated pregnancy outcomes and the influence of pregnancy in patients with Brugada syndrome.
MethodsA retrospective analysis was performed in all pregnant women with Brugada syndrome. We included 104 women with a total of 219 deliveries.
ResultsThere were 15 spontaneous abortions. One infant died suddenly during the night 3 months after birth. Six pregnant women reported they had experienced at least 1 syncope during the pregnancy. Of the 3 women who received an implantable cardioverter-defibrillator before the pregnancy, none received arrhythmia episodes. There were no events during the pregnancy in 4 patients with a previously aborted sudden cardiac death. Of 24 patients with syncope when not pregnant, 18 were asymptomatic and 6 experienced a recurrent syncope during the pregnancy. During the follow-up (mean follow-up 298.9 days; 95% confidence interval, 289.6-308.2), 2 women received appropriate shocks.
ConclusionsIn this retrospective, single-center study, serious events were not more frequent during pregnancy and the peripartum period in women with Brugada syndrome. The occurrence of syncope during pregnancy was not associated with a worst outcome in the peri- and postpartum periods or during follow-up. The reported rate of miscarriage and sudden infant death will require further studies to confirm or rule out its association with Brugada syndrome.
Keywords
The sex-related difference in the phenotypic expression of the Brugada syndrome (BS) is more pronounced than in any other autosomally transmitted arrhythmic syndrome.1,2 The basis for this intriguing sex-related distinction is not fully understood. Potential explanations are gender-related intrinsic differences in ionic currents and hormonal influences. Due to this hormonal influence, pregnancy represents a particular situation in the life of women with BS. To date, data elucidating the role of hormonal changes secondary to pregnancy in the clinical outcome of this population have been missing. In 1 isolated case report, pregnancy was suggested to be a precipitating factor for triggering electrical storm.3 The aim of our study was to evaluate the frequency of clinical events during pregnancy and the peripartum period.
METHODSStudy PopulationPatients were consecutively included in the current study from our database if they met the following inclusion criteria: female sex, age between 18 and 65 years, documented spontaneous or class I antiarrhythmic drug-induced coved type 1 BS electrocardiogram (ECG), and previous deliveries. Those patients who were not followed-up in our institution were contacted through telephone interview. During the interview, several factors were evaluated: the number of deliveries, cesarean or spontaneous delivery, the number of spontaneous abortions, potential arrhythmic events during the pregnancy (syncope, arrhythmias or sudden cardiac death [SCD]), and the status of the newborn (healthy child, sudden infant death).
A proband was defined as the first patient of a family with BS diagnosed with type 1 Brugada ECG pattern. Diagnosis of BS was based on the recommendations of the Brugada consensus reports4,5 with recent modifications.6,7 Syncope was defined as transient loss of consciousness with abrupt onset and offset.
All included patients gave their informed consent. The Ethics Committee of the UZ Brussels (Brussels, Belgium) approved the study protocol. The database for the purpose of this study was assessed in August 2012.
Statistical AnalysisContinuous variables are expressed as the mean and standard deviation or median. Statistical differences were calculated using the chi-square test for discrete variables and the t-test for continuous variables (expressed as mean values and standard deviation). Cumulative event rates were estimated by Kaplan-Meier estimation. A P-value<.05 was considered statistically significant. All statistics were performed with the use of SPSS software (SPSS Inc., Chicago, Illinois, United States).
RESULTSOne hundred and four women (belonging to 79 families) with a total of 219 deliveries were included from a total of 611 individuals in the original database (from 1995 to 2012). Two pregnant women were still pregnant at the time of the interview and were excluded from the analyses. One patient was lost to follow up. Five patients were diagnosed with BS before pregnancy and the remainder of the sample was diagnosed afterward.
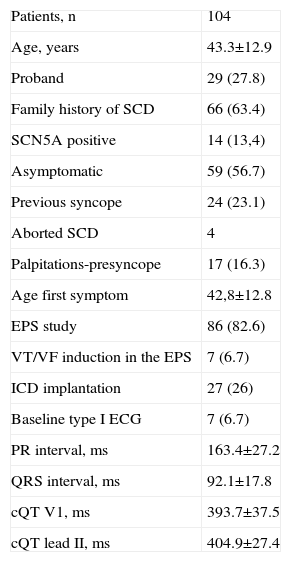
The mean age of the sample at diagnosis of BS was 43 (12.9) years. Twenty-nine women were probands. Sixty-six individuals had a positive family history of SCD (Table 1). The mean age at the first symptom was 42 (12.7) years. Fifty-nine patients were classified as asymptomatic, while 24 had had a previous syncope, and 4 had an aborted SCD as the first manifestation of the disease. Baseline ECG showed a spontaneous type 1 BS pattern in 7 patients, a normal pattern in 58 patients, and type 2 and 3 pattern in 39 women. Eighty-six women underwent an electrophysiological study (EPS). A sustained ventricular arrhythmia was induced in 7 study patients. Twenty-seven patients received an implantable cardiac defibrillator (ICD); the reason for the implantation was based on several characteristics but can be summarized as follows: the presence of an aborted SCD (4 women), a history of previous syncope (20 patients, 4 of them with positive EPS), and positive EPS in patients with a positive ajmaline test (3 patients). The mean age was 25 (2.9) years at the first delivery, 27 (3.4) at the second, 31 (3.1) at the third, and 28 (16) at the fourth. There were 5 patients with 4 deliveries, 2 patients with 5, and 1 patient with 6. Thirteen women underwent cesarean section and the remaining newborns were delivered vaginally.
Baseline Characteristics of the Population Enrolled in the Study
| Patients, n | 104 |
| Age, years | 43.3±12.9 |
| Proband | 29 (27.8) |
| Family history of SCD | 66 (63.4) |
| SCN5A positive | 14 (13,4) |
| Asymptomatic | 59 (56.7) |
| Previous syncope | 24 (23.1) |
| Aborted SCD | 4 |
| Palpitations-presyncope | 17 (16.3) |
| Age first symptom | 42,8±12.8 |
| EPS study | 86 (82.6) |
| VT/VF induction in the EPS | 7 (6.7) |
| ICD implantation | 27 (26) |
| Baseline type I ECG | 7 (6.7) |
| PR interval, ms | 163.4±27.2 |
| QRS interval, ms | 92.1±17.8 |
| cQT V1, ms | 393.7±37.5 |
| cQT lead II, ms | 404.9±27.4 |
ECG, electrocardiogram; EPS, electrophysiological study; ICD, implantable cardiac defibrillator; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
Data are expressed as No. (%) or mean±standard deviation.
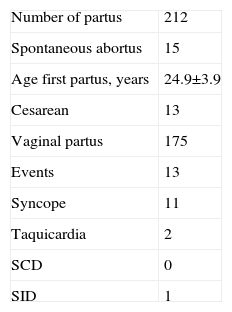
During pregnancy, there were 15 spontaneous abortions in 10 patients (5 women with 2 spontaneous abortions and 5 with 1) (Table 2). This subgroup showed no differences in clinical characteristics compared with those in patients without spontaneous abortions (Table 3). Two patients complained of recurrent tachycardia but without ECG documentation. Six women reported at least 1 syncope during pregnancy (in 2 of them, the syncope recurred in the following deliveries, with a total of 11 episodes); in 3 women, the first syncope occurred during the study period.
Description of Deliveries and Events During Pregnancy in the Study Sample
| Number of partus | 212 |
| Spontaneous abortus | 15 |
| Age first partus, years | 24.9±3.9 |
| Cesarean | 13 |
| Vaginal partus | 175 |
| Events | 13 |
| Syncope | 11 |
| Taquicardia | 2 |
| SCD | 0 |
| SID | 1 |
SCD, sudden cardiac death; SID, sudden infant death
Data are expressed as No. or mean±standard deviation
Baseline Characteristics of Patients With Brugada Syndrome According to the Presence or Absence of Spontaneous Abortions
| Spontaneous abortions (n=10) | No abortions (n=94) | P values | |
| Patients, n | 15 | 219 | |
| Age first delivery, years | 25 | 25 | .8 |
| Syncope, n | 4 | 17 | .2 |
| SCD, n | 0 | 4 | .4 |
| Family history SCD, n | 7 | 59 | .6 |
| Baseline type 1, n | 1 | 6 | .6 |
| ICD implantation, % | 3 | 23 | .9 |
| Positive genetic test, n | 0 | 14 | .4 |
ICD, implantable cardiac defibrillator; SCD, sudden cardiac death.
None of the women with an ICD prior to the pregnancy (n=3) received ICD therapy during the study period. Those patients with aborted SCD prior to the pregnancy had no events during the pregnancy. Conversely, of the 24 women with syncope outside the setting of the pregnancy, 18 were asymptomatic during the pregnancy. Patients with a positive genetic test (SCN5A) (n=14) had no more syncope before or during delivery (P>.7). No serious adverse events were reported during the peripartum period. One infant died suddenly during the night 3 months after delivery.
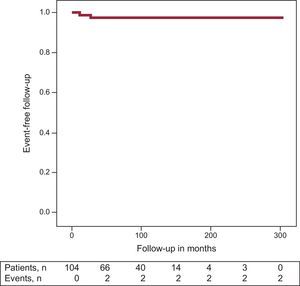
Follow-upDuring the follow-up (mean follow-up 298.9 days, 95%CI, 289.6-308.2), 2 women had ≥1 appropriate shock; 1 patient received the shock 11 months after ICD implantation and the second 26.9 months later (the first patient had a positive result in the EPS, the second did not undergo an EPS because she had been resuscitated from an SCD and consequently EPS was not performed). In both women, the pregnancy was successful, with no events during pregnancy and normal fetal outcome. In a third patient, runs of nonsustained ventricular tachycardia were recorded in the ICD. Event-free survival at 3 years was 97.6% (Figure) (this event rate refers to follow-up in months after diagnosis of BS and not from the pregnancy period). Two patients developed atrial fibrillation in the follow-up.
Among the 6 patients with syncope during the pregnancy, 1 of them continued to have recurrent episodes after delivery. An ajmaline test was performed unmasking a type 1 ECG BS. In the EPS, a ventricular fibrillation was induced and an ICD was then implanted. The patient had no further episodes and no events were recorded in the ICD. Four patients continued to experience syncope after delivery. These patients had a family history of SCD. Three of them underwent ICD implantation. The sixth patient, a relative of a patient with BS and with a positive ajmaline test, also experienced syncope after delivery but, due to the vasovagal profile, no ICD was implanted and no events were documented during long-term follow-up.
Seven patients received inappropriate shocks (because of lead fracture or fast atrial fibrillation) and 1 patient required ICD removal due to lead infection.
DISCUSSIONIn this retrospective, single-center study including 104 women with BS and previous deliveries, there were 15 miscarriages in 10 women, 6 women experienced recurrent syncope during the pregnancy, no serious events were reported during the peripartum period, and 1 infant died suddenly at the age of 3 months. Pregnancy was complete with no marked disturbances in patients with an ICD implantation prior to delivery. Finally, the event-free rate at 3 years after diagnosis of BS was 97.6% (1.7).
The syndrome of right bundle branch block, ST-segment elevation and SCD, known as BS, was first described in 1992 as a new clinical and electrocardiographic syndrome involving susceptibility to ventricular arrhythmias and SCD in patients without obvious structural heart disease.8 Of note, the sex-related difference in the phenotypic expression of the syndrome is more pronounced than in any other autosomally-transmitted arrhythmic syndrome and the manifestation of the clinical syndrome is observed 10 times more often in males than in females.9,10
The basis for this intriguing sex-related distinction is not fully understood. Specific mutations seem to be of minor relevance as patients with and without identified SCN5A mutations display a similar male predominance.11,12 Other hypotheses and explanations consist of sex-related intrinsic differences in ionic currents and hormonal influences. Regarding the former, Di Diego et al.1 provided insights into the cellular and ionic bases for sex-related distinctions, showing that the presence of a more prominent Ito in males underlies their predisposition to the development of the Brugada phenotype. They reported clear differences in Ito density and inactivation kinetics in RV epicardial cells isolated from male vs female canine hearts. Conversely, there are suggestions that hormonal effects may play a role in the phenotypical differences between sexes. Likewise, disappearance of the ECG type I pattern has been reported after castration because of prostate cancer in patients with BS.13 Moreover, testosterone concentrations seem to be significantly greater in men with BS than in controls.14 Some experimental studies suggest that hormones could exert their effect by modifying ion currents.15,16 Thus, estrogen suppresses the expression of the Kv4.3—the gene coding for Ito—resulting in reduced Ito and a shallow phase 1 notch. On the other hand, testosterone enhances the outward currents (IKr, IKs, and IK1) and reduces the inward current (ICa-L), thus deepening the phase 1 notch.14,17 In an experimental study, Kv4.3 has been shown to be down regulated, resulting in reduced Ito in rat myometrium at the end of pregnancy caused by a rise in estrogen levels.16
Due to this hormonal influence, pregnancy represents a particular situation in the life of women with BS. To date, no studies have been reported in this population with BS. Sharif-Kazemi et al., 3 reported a case of BS revealed during pregnancy in a young woman who presented with electrical storm as the first manifestation. The authors hypothesized that elevated hormonal levels in pregnancy might be a precipitating factor for triggering electrical storm in some cases. Ambardekar et al.18 suggested that elevated testosterone levels during pregnancy attributable to estrogen-induced increases in hepatic synthesis of sex hormone-binding globulin might play a role. In our study, 6 women had recurrent syncope during the pregnancy and no single patient experienced serious events during delivery.
Syncope represents one of the most relevant risk markers in patients with BS. Nonetheless, it lacks specificity and may be due to different reasons, such as an impaired autonomic nervous system balance, sinus bradycardia, sinoatrial conduction abnormalities, prolonged HV interval, AV block, AV nodal reentrant tachycardia, atrial fibrillation, and polymorphic ventricular tachycardia. During pregnancy, syncope is one of the most common complaints, together with palpitations, dizziness and presyncope.19 However, the cause of syncope during pregnancy remains unclear in most cases. Shotan et al.19 failed to show a positive association between symptoms and ectopic activity in their study conducted in 110 consecutive pregnant patients with no evidence of heart disease referred for evaluation of palpitations, dizziness, and syncope. The presence of arrhythmias could be documented in only 10% of symptomatic episodes. In the present cohort, syncope was not associated with complications during the pregnancy or with events in the follow-up. However, to date, the presence of previous syncope is considered a marker of high risk for future ventricular arrhythmias in patients with BS and must therefore be taken into account in risk stratification.
The rate of pregnancy loss among clinically diagnosed pregnancies has been reported to be between 8% and 15%.20 These percentages are concordant with the data reported here. Nonetheless, given the particular profile of the study population and that approximately 50% of early pregnancy losses are caused by chromosomal abnormalities,21 unusual forms of different channelopathies with extremely severe manifestations during the first months of pregnancy could be a plausible cause of spontaneous abortions.
In patients with BS and an ICD, no noteworthy problems were reported during the pregnancy or the peripartum period. Although Schuler et al., 22 reported a considerable rate of complications in pregnant women with heart disease and an ICD, Natale et al.23 found no increases in device or treatment complications, nor any increase in the number of shocks the women received compared with preconception.23 Our data (although based on only 3 women with an ICD before pregnancy) suggest that women with ICDs and BS successfully negotiate pregnancy. However, we routinely take precautions to ensure a safe outcome. During pregnancy, heart rate increases by 25%; thus sinus tachycardia, particularly in the third trimester, is not uncommon.24 For this reason, in our center, 1 month before the expected delivery, ventricular fibrillation detection was programmed to 230 bpm. In programmed cesarean deliveries, fast access to an ICD programmer was prearranged, as well as cardiological supervision.
Concerning infant status, there was 1 infant who died suddenly 3 months after birth. Importantly, distinct channelopathies, such as BS25 and long-QT syndrome,26 have been reported to have caused some cases of sudden death in children even in the first months of life when these channelopathies may be misdiagnosed as sudden infant death syndrome (SIDS). In 1998, Schwartz et al.26 reported the results of a 19-year prospective study of more than 34 000 infants who underwent electrocardiography on the third or fourth day of life. These authors found that 50% of the infants who died of SIDS had a prolonged QT interval corrected for heart rate (QTc). BS has also been associated with SIDS but, possibly due to the intermittent nature of the ECG pattern, correct clinical diagnosis may be challenging. In the initial report on BS, 3 of the 8 patients were children.8 Priori et al.25 reported the cases of 5 children from the same family who died after unexplained cardiac arrest: 2 of them had been previously classified as SIDS. BS was suspected based on the transient manifestation of the typical ECG pattern in 1 of the children. Thereafter, a mutation in the cardiac sodium-channel confirmed the diagnosis of BS, which suggests that this disease may be the cause of sudden death in children. To the best of our knowledge, there are no reports of the rate of SIDS in a series of related patients with BS and, although based on a limited number of cases, it is our belief that this topic merits further investigation to confirm or rule out its association with BS.
Finally, event-free survival at 3 years after diagnosis of BS was 97.6%, as we previously reported.10 Although the aim of our study was not to compare the rate of events between men and women, this rate appears to be lower during follow-up in women than that reported for men.27
To sum up, pregnancy represents a particular status in women with BS. Spontaneous delivery was safe in this population, including those patients with previous ICD. Further investigation on spontaneous abortions and SIDS is necessary to assess whether these events are related to the disease. Likewise, the management of these patients involves particular considerations that should be taken into account to ensure a favorable maternal and fetal outcome.
LimitationsThe results of our study should be considered in light of its potential limitations. First, an important limitation of this study is the inclusion criterion of a previous delivery, which could have introduced a bias in the results, since pregnant women with BS and sudden death during pregnancy would not have been included in the study group. Thereafter, lethal ventricular arrhythmias may be underrepresented in this series.
Second, our study has the obvious limitation of being retrospective. Importantly, however, all patients, except 1, were contacted and their current status checked, so the bias introduced by patients lost to follow-up was cancelled out.
Another limitation is that the study population may have been influenced by referral bias, because our hospital is a referral center for BS. Our unit may attend a higher percentage of patients with a family history of BS than probands compared with other units. However, we do not believe that this possibility alters the conclusions of this study.
The presence of a family history of SCD is at times difficult to assess and is occasionally based on patients’ memories rather than on a medical report, which could have introduced a bias that tends to overestimate the perception of sudden death attributable to a cardiac origin.
Finally, because some patients were contacted many years after the pregnancy, some details (events during pregnancy and syncope) could have been forgotten, which was the main reason why patients older than 65 years were excluded from the database.
CONCLUSIONSIn this retrospective, single-center study, serious events were not more frequent during pregnancy and the peripartum period in women with BS. The occurrence of syncope during pregnancy was not associated with a worse outcome during the peri- and postpartum period or during follow-up. The reported rate of miscarriage and sudden infant death requires further studies to confirm or rule out their association with BS.
CONFLICTS OF INTERESTDr. Rodríguez-Mañero received a post-residency grant for international research from the European Heart Rhythm Association (EHRA Training Fellowship).