Antithrombotic therapy is the fundamental treatment for various cardiovascular conditions, e.g. ischemic heart disease, atrial fibrillation (AF), and stroke to prevent thrombotic complications and death. Dual antiplatelet inhibition with acetylsalicylic acid (ASA) and a P2Y12 inhibitor has proven most effective in patients with recent myocardial infarction (MI) or after percutaneous coronary intervention (PCI),1–3 whereas oral anticoagulation with vitamin K antagonist (VKA) or newer oral anticoagulants (NOACs: rivaroxaban, apixaban, or dabigatran) is most effective in AF.4–7 Long-term oral anticoagulation with VKA is also recommended in patients with mechanical heart valves.
The safety and efficacy of different antithrombotic regimens have been assessed in randomized trials exploring treatments in patients with a unanimous indication for antithrombotic treatment, such as acute coronary syndrome (ACS) or AF.1–3,5–7 However, the need for antithrombotic protection can change over time due to temporal changes in the patient's baseline risk, eg, after PCI, insertion of an artificial heart valve, occurrence of venous thromboembolism, or stroke. Approximately 6% to 8% of patients with ACS have an indication for oral anticoagulation, whereas 20% to 30% of patients with AF have co-existing ischemic heart disease. Consequently, the combination of dual-antiplatelet therapy and oral anticoagulation is frequently requested.8,9 Treatment with triple therapy, defined as treatment with ASA, a P2Y12 inhibitor and oral anticoagulation, can be indicated in some patients but usually for a limited period of time, the most typical clinical situation being a patient with a clear indication for oral anticoagulation experiencing an acute MI or treatment with PCI. The combined use of oral anticoagulation and antiplatelet therapy increases the risk of bleeding, whether considering triple therapy with VKA, ASA and clopidogrel or triple therapy with a NOAC.10–14 The knowledge and experience with NOACs in triple therapy is very sparse, and as co-existing ischemic heart disease is common in patients with AF, the bleeding risk related to triple therapy with NOACs is a major clinical question. Occurrence of bleeding is followed by an increased risk of thrombosis and death, thus triple antithrombotic therapy should be limited to the shortest possible time period, and should always be preceded by an individual assessment of bleeding risk.15
INDICATIONS FOR DUAL ANTIPLATELET THERAPYIn patients with MI and PCI, dual antiplatelet treatment with ASA and clopidogrel has been recommended since 2001 to prevent complications such as stent-thrombosis, recurrent MI, and stroke.1 Later on, two other P2Y12 inhibitors, prasugrel and ticagrelor, were developed and made available in clinical practice.2,3 Prasugrel and ticagrelor have proven more effective than clopidogrel in ACS, with less variation of platelet inhibition, and are now recommended as preferred P2Y12 inhibitors after MI.2,3,9
ANTICOAGULATIONAnticoagulation is recommended in AF patients with high risk of stroke, the risk being easily assessed by the use of a risk stratification scheme such as the CHA2DS2VASc score, which includes various baseline characteristics (congestive heart failure or left ventricular dysfunction, hypertension, age≥75 years [double score], diabetes, stroke [double score], vascular disease, age 65-74 years, sex category [female]). Anticoagulation is recommended in most patients with CHA2DS2VASc score≥1 (except patients receiving 1 point for female sex), but can also be indicated in other conditions such as venous thromboembolism or in patients with prosthetic heart valves.4,15 Previously, VKA was the preferred oral anticoagulation, but since the introduction of the NOACs, these are now recommended as first-line therapy in most patients with AF, due to a more favorable side effect profile with lower risk of intracerebral bleedings.15 For patients with venous thromboembolism, rivaroxaban or VKA can be used, whereas VKA at present is the only choice for patients with prosthetic heart valves.
TRIPLE ANTITHROMBOTIC THERAPYThe efficacy and safety related to triple therapy have primarily been described by observational studies, or smaller randomized trials, and most studies have described combinations of ASA, clopidogrel, and VKA.10,11,16,17 Only a few studies have examined combinations of ASA, clopidogrel, and NOAC,12,14,18 and one study the combination of ASA, prasugrel, and VKA.19 However, these studies are difficult to compare due to differences in the study populations (stable and unstable patients, difference in age and severity of comorbidity), and use of different bleeding definitions and outcomes.
Triple Therapy: Acetylsalicylic Acid, Clopidogrel, and Vitamin K AntagonistThe crude incidences of major bleedings in triple therapy studies (ASA, clopidogrel, and VKA) range from 5% to 15%, most being greater than 10% per year.10,11,16,17 It has been more difficult to establish concordance of bleeding risk related to dual therapy with a VKA and either ASA or clopidogrel.10,16,17,20 Two large-scale, nationwide, observational studies have found nearly as high risk of bleeding related to treatment with VKA and clopidogrel as with triple therapy, but a recently published randomized trial of 573 patients treated with PCI (the What is the Optimal antiplatElet and anticoagulant therapy in patients with oral and coronary StenTing [WOEST] trial), with an indication of oral anticoagulation showed reduced risk of bleeding in patients receiving VKA treatment combined with clopidogrel compared with triple therapy (6.5% vs 12.7%, P=.01).10,21 To our knowledge, the WOEST trial is the first randomized trial testing the safety of triple therapy against dual treatment with VKA and clopidogrel. In the study, the incidence of bleeding was relatively high (triple therapy 15.8%, VKA and clopidogrel 6.5%), but not markedly different from real-life patients in large observational studies.10,11,21 It is noteworthy that the trial found lower all-cause mortality in the group treated with VKA and clopidogrel compared with triple therapy, which could be explained by a lower number of bleedings, and a subsequent lower risk of thrombosis and death.11 There are no randomized studies of triple therapy that have been powered to test mortality and frequency of stent thrombosis. Two observational studies found comparable risk of thrombotic events in triple therapy compared with VKA combined with one antiplatelet drug (ASA or clopidogrel), but did not evaluate stent-thrombosis.16,21 The WOEST trial was not powered to detect differences in rates of mortality or thrombosis (main interest being death, stent-thrombosis, and recurrent MI), but the number of events was reassuring, with a reduced all-cause mortality of 2.5% in the dual treatment group, compared with 6.3% in the triple treatment group; P=.03) and no increase in recurrent MI, stroke, or stent-thrombosis in the dual treatment group.11
Triple Therapy: Acetylsalicylic Acid, Prasugrel, and a Vitamin K AntagonistOne study has described the use of prasugrel in triple therapy, and none have described ticagrelor in triple therapy.9,19 The use of prasugrel in triple therapy with ASA and VKA was compared with triple therapy including ASA, clopidogrel, and VKA (n=377, 21 patients received prasugrel). Prasugrel was given in one of the following situations: a) high platelet reactivity (assessed by function testing); b) high risk of stent-thrombosis (comorbidities, complexity of lesion, stent-thrombosis on clopidogrel treatment); c) ACS, where the patient had not switched to clopidogrel, and d) clopidogrel allergy. Bleedings occurred in 28.6% of those receiving prasugrel-triple therapy and 6.7% of those receiving clopidogrel-triple therapy, and confirmed increased risk in the adjusted analysis (hazard ratio [HR]=3.2; 95% confidence interval [95%CI], 1.1-9.1; P=.03). There was no difference in thrombotic events.
Triple Therapy: Acetylsalicylic Acid, Clopidogrel, and Novel Oral AnticoagulantsTriple therapy with rivaroxaban, apixaban, or dabigatran etexilate combined with ASA and clopidogrel has been tested in patients with ACS to explore whether a prolonged anticoagulation could reduce the residual risk of thrombosis in patients with MI.13,14,22 Apixaban was added to ASA and clopidogrel in therapeutic doses (5mg twice daily), whereas rivaroxaban was used in lower doses than recommended for oral anticoagulation (2.5mg or 5.0mg daily). Dabigatran was tested in a phase II trial at different doses (50mg, 75mg, 110mg, 150mg twice daily).13,14,22
Rivaroxaban in Triple TherapyThe trial testing triple therapy of rivaroxaban, ASA, and clopidogrel against ASA and clopidogrel used rivaroxaban in lower than therapeutic (anticoagulant) doses, as a phase-II dose-finding trial found dose-dependent increase of bleedings with higher doses (bleeding rates being 15.3% for 20mg, 12.7% for 15mg, 10.9% for 10mg 6.1% for 5mg, and 3.3% for placebo).18 The outcome of the study was: reduced combined endpoint of cardiovascular death, MI, or stroke in the groups receiving rivaroxaban, but increased TIMI (Thrombolysis In Myocardial Infarction)-major bleedings (2.1% with rivaroxaban vs 0.6% with placebo; P<.001). The rates of intracerebral bleedings with rivaroxaban were 0.6% vs 0.2% with placebo. In addition, rivaroxaban 2.5mg reduced all-cause mortality.13
Apixaban in Triple TherapyThe trial testing triple therapy of apixaban, ASA, and clopidogrel against ASA and clopidogrel used full anticoagulant doses of apixaban (5mg twice daily). The primary efficacy endpoint was a combined of cardiovascular death, MI, or stroke, and the safety endpoint major bleeding (TIMI-major definition). The study was stopped prematurely after randomization of approximately 7000 patients, due to excess of bleedings in the apixaban group (1.3% in the apixaban group vs 0.5% in the placebo group; P=.001), without benefit on the efficacy endpoint.14
Dabigatran in Triple TherapyDabigatran was studied in ACS in a phase II trial, which showed more bleedings among patients treated with dabigatran (dabigatran dose-dependent increased bleedings: 50mg, 3.5%; 75mg, 4.3%; 110mg, 7.9%, and 150mg, 7.8%).22 A phase III trial has not been initiated. However, additional use of antiplatelet therapy was allowed in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) study, where 38.4% of the patients at some point used antiplatelet therapy during the study-period. A substudy analysis confirmed the results from the main analysis, that dabigatran 110mg twice daily was noninferior to warfarin, with fewer bleedings irrespective of concomitant antiplatelet use. In patients with dabigatran 150mg, the efficacy endpoint occurred less frequently in patients without antiplatelet therapy, with a reduced benefit among those taking antiplatelet therapy. Time-dependent analysis showed increased risk of bleeding associated with antiplatelet use, a risk that was accentuated with the number of antiplatelet drugs used (HR=2.31; 95%CI, 1.79-2.98 for dual-antiplatelet, and HR=1.60; 95%CI, 1.42-1.82 for single antiplatelet, no antiplatelet as reference).7,12 Since these data are based on a substudy and not predefined in the study protocol, interpretation of these results should be cautious.
TRIPLE ANTITHROMBOTIC THERAPY: SUMMARY AND DISCUSSIONSummaryUse of triple therapy can be indicated for limited time periods to prevent serious thrombotic events such as stroke, recurrent MI, stent-thrombosis, and death. Triple therapy should only be used during the period of time where the risk of thrombosis is high; at present a maximum of 12 months is recommended for patients with an indication of oral anticoagulation and experiencing MI.8,9,15
All combinations of oral anticoagulants (VKA, rivaroxaban, apixaban, or dabigatran), ASA and a P2Y12 inhibitor (clopidogrel or prasugrel) increases the risk of bleeding, but direct comparison of bleeding rates is difficult due to variations in study design and outcomes. Increased number of antithrombotic drugs used in combinations accentuates the risk of bleeding, as does increased dose of NOACs.10,12–14,18,21,22
A bleeding episode is followed by an increased risk of thrombosis and death, which might explain the beneficial effect on all-cause mortality of low-dose rivaroxaban (2.5mg) when used in combination with ASA and clopidogrel in patients with ACS, but not in the high doses, and the reduced mortality found in the WOEST trial among patients receiving dual therapy with VKA, compared with patients in triple therapy (both with lower number of bleedings).
RecommendationsIn clinical settings where triple therapy is indicated, the following combinations are recommended by the European Society of Cardiology: use of VKA or dabigatran 110mg, combined with ASA and clopidogrel.8,9,12,15 Rivaroxaban has not been tested in triple therapy in doses recommended for complete anticoagulantion.13 Triple therapy including apixaban (ASA and clopidogrel) or prasugrel (ASA and VKA) cannot be recommended at present, due to increased risk of bleeding.14 The bleeding risk associated with ticagrelor in triple therapy is unknown and thus cannot be recommended.
Results from the WOEST trial show that dual-therapy with VKA and clopidogrel is associated with reduced risk of bleeding, with no increased risk of thrombosis, but the study was not powered to detect differences in stent-thrombosis, for example. The results of the WOEST trial have been confirmed in a large observational study; the results are reassuring and support the anticipation that clopidogrel and VKA can be used as a safer combination in patients at high risk of bleeding.8,11
Studies on efficacy and safety of ticagrelor or prasugrel and NOACs in triple therapy are urgently warranted, since the knowledge on these combinations is sparse.
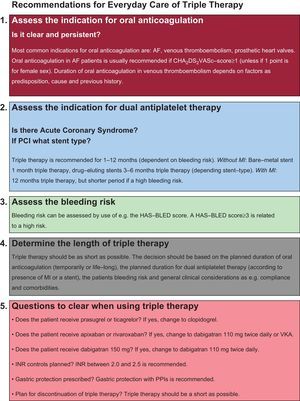
EVERYDAY CARERisk Assessment, Duration, Proton-pump Inhibitors, and International Normalized RatioIn everyday care, focus on safety is a key-element when considering a patient for triple therapy. Triple therapy should be planned for the shortest possible duration with focus on presence or absence of ACS, individual assessment of bleeding risk, and type of stent.8,9 The use of proton-pump inhibitors in ACS has been debated heavily, due to suspected interactions, but both observational studies and randomized trials have shown comparable risk of thrombotic events among those treated with or without proton-pump inhibitors while receiving a P2Y12 inhibitor (clopidogrel or prasugrel).9 Gastric protection with proton-pump inhibitors is recommended in patients receiving more than one antithrombotic agent, if predictors of upper gastrointestinal bleeding are present (nonsteroidal anti-inflammatory drugs, steroids, oral anticoagulation, previous gastrointestinal bleeding, and patients with Helicobacter pylori infection).8,9 Variance of the international normalized ratio (INR) values in patients treated with VKA is a predictor of increased risk of bleeding (INR>2.6).8 It is recommended in AF patients treated with VKA and dual antiplatelet therapy that target INR be kept between 2.0 and 2.5.8Figure shows a checklist that can be used in everyday care for patients considered for triple therapy.
CONCLUSIONSTriple therapy with dual antiplatelet therapy and oral anticoagulation can be indicated in certain clinical situations for a limited period of time to prevent serious thrombotic events. Recommended duration of triple therapy is dependent on presence or absence of ACS, risk of bleeding, and type of stent (in patients treated with PCI). Triple therapy increases the risk of bleeding, and continuous evaluation of the appropriateness of current antithrombotic treatment is highly important, as bleeding is associated with a higher subsequent risk of thrombosis and death. Triple therapy should be kept as short as possible, and include oral anticoagulation of VKA or dabigatran 110mg twice daily, ASA, and clopidogrel.
CONFLICTS OF INTERESTNone declared.