Torrent Guasp's theory of heart function is based on grouping the muscle fibers that make up the ventricular myocardium in a continuous band coiled in a double helix.1,2
The different parts of this band cannot be identified by direct observation with optical instruments or by palpation. The dissection performed by Torrent Guasy and those who learnt from him reproduces the same structure in all hearts, and this reproducibility is the only objective proof of the original theory that the band exists as an anatomical structure.
The dissection techniques used by many cardiologists and investigators (fingertip dissection in previously boiled hearts) do not identify pre-existing separation planes, and the end result is an artefact that is similar to what happens when craftwork is reproduced: a preconceived model is copied and the artist's handiwork makes every piece identical.
In general, for physiologists and biophysicists, the myocardial arrangement as a band with 2 helices greatly facilitated their understanding of cardiac mechanics and of the efficiency of the heart to generate pressure.3,4 Much of the evidence that has contributed to showing that the band is the true anatomical substrate of the heart comes from this “physiological convenience”. There is maximum interdependence between structure and function in the heart, and cardiac output can hardly be explained without an appropriate structure or accepting the simple anatomy required for competent development of cardiac function, although this greatly hinders its study.5–9 The article published in Revista Española de Cardiología by Poveda et al.10 shows images that would appear to represent myocardial fibre tracts in situ, with the same form and complexity as that of the band once dissected, arranged and “set up” in a suitable position. However, these snapshot images have been obtained by software that processes magnetic resonance (MR) signals derived from the conductivity of the water molecules between the myocardial fibers of ex vivo dog hearts. Therefore, it could be said that they are the shadow of the fibers and some highly sophisticated anatomical parts; however, the most interesting feature is their suggestive nature.
WHAT IS CARDIAC DIFFUSION TENSOR MAGNETIC RESONANCE IMAGING?Diffusion tensor imaging studies the diffusivity of water molecules. The information about the tract of myocardial fibers comes from the principle that the main orientation of myocardial microstructures is parallel to the direction of maximum diffusion of the water molecules, whose signal is attenuated in the presence of a magnetic gradient.11 Thus, in each myocardial pixel, MR provides 3 orthogonal vectors (eigenvectors) and their magnitudes (eigenvalues). By means of calculations and mathematical models, 3 important parameters are obtained in order to define the myocardial fibers: fractional anisotropy, mean diffusivity, and helix angle.
Mean diffusivity is calculated as the average of 3 eigenvectors, and any alterations reflect pathological changes that modify the redistribution of intracellular or extracellular volume.
Fractional anisotropy is the degree of deviation that an ellipsoid shows with respect to a sphere, which is isotropic. It is calculated from the normalized standard deviation of the 3 tensor eigenvectors using their magnitudes. This parameter measures variability in water mobility in different directions; it decreases in the presence of disorganized tissue structure. It is related to tissue architecture and provides a measure of tissue integrity. A value of 0 indicates that diffusion is isotropic (in all directions) and a value of 1 indicates anisotropic diffusion, occurring in only one direction.
The orientation of the fibers in each pixel is defined as the principal eigenvector of the tensor and the helix angle of the fiber is the angle between the local fiber vector and the local circumferential vector (the circumferential vector is perpendicular to both the longitudinal axis of the left ventricle and to the local radial vector) and it represents the fiber architecture.
Diffusion tensor MR imaging characterizes the tissue and provides information on fiber architecture. It was originally used in neurosurgery to select which tissue to resect. With regard to the heart, a few studies have been published on the identification of tissue affected by myocardial infarction,12 but it is very difficult to obtain valid images due to heart and respiratory movements.13
The images presented in the paper by Poveda et al.10 are taken from dog hearts submerged in a substance that is ideal because of its low sensitivity to magnetism. The experimental source data were taken from a cardiac MR imaging database. The authors compared the diffusion tensor MR images with the arrangement and structure of the various segments of the myocardial band folded in its anatomical position in a rubber mould designed by Torrent Guasp. The different parts of the band coincide in their position in the myocardium and in the direction they follow. The nonsimplified images in Figures 2 and 3 in the article by Poveda et al.10 show the band coiled in its anatomical position. The transparency shows the descending segment forming the septum and apex in the mesocardium. It is also surprising to see the twist in the simplified images that occurs at the start of the descending apical segment and how it enters the subendocardium to form the septum and apex.
These MR images were obtained in explanted hearts and therefore there is no possible reference to cardiac cycle phases. The big question is whether diffusion tensor images can provide information about heart dynamics. Specifically, whether the orientation of the vectors presented in Figures 1A and 1B in the paper by Poveda et al.10 means that water diffusion takes place mainly or only in one direction and whether that direction is related to the mechanical activity of the heart. The authors deduce that water diffusion can occur in both directions and that the different orientation of the vectors does not correspond to one particular coil—since one coil is inside the other in the double helix—and the direction of contraction may correspond to different coils depending on the angle cut.
FUNCTIONAL EVIDENCE OF THE EXISTENCE OF A COILED BAND STRUCTURESome investigators have tried to provide functional evidence that would necessarily imply the presence of a functional band.3,5,8 Torrent Guasp suggested that the contractile wave would travel along the band from the subpulmonary area where the band started, to the aortic root, where it ended.1,2 Thus, several investigators used different techniques to try to provide evidence of this sequential contraction. In our laboratory, we used sonomicrometry to establish this sequence by measuring the start of contraction at 4 points in the ventricular myocardium, identifying them with 4 matching points along the muscle band trajectory: a) epicardium in the subpulmonary area (identifying the right segment of the basal loop); b) lateral wall of the left ventricle (identifying the left segment of the basal loop); c) mesocardium of the anterior area of the left ventricular apex (identifying the descending segment of the apical loop), and d) epicardium in the subaortic area (identifying the ascending segment of the apical loop).
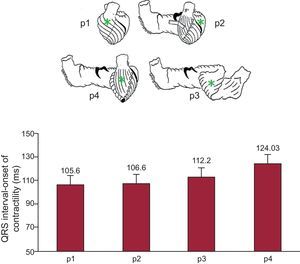
Figure 1 shows the times between the start of the QRS complex and the onset of contraction at these 4 points, measured using pairs of implanted ultrasonic crystals. This progressive contraction along the band implies an additional quality step; the structure is linked to a particular direction of contraction, which means that the band arrangement and this sequential form of contraction are responsible for cardiac function.
Experiments in open-chest pigs (n=32). The illustrations at the top show the uncoiled myocardial band according to Torrent Guasp. The points marked (p) show where the pairs of ultrasonic crystals have been implanted, following the direction of the fibers. The graph at the bottom shows mean (standard error) values for the times of onset of contraction at the points described above. The subpulmonary area and left ventricular free wall are the areas with the earliest contraction, followed by the mesocardium on the anterior aspect of the apex, which corresponds to the descending segment of the band. Finally, the epicardium contracts on the anterior aspect of the left ventricle corresponding to the ascending segment of the apical loop (own unpublished data).
The functional properties of myocardial fibers can be summarised as follows: a) longitudinal contraction leads to increased thickness perpendicular to the shortening process; b) the electrical activation of a fiber is transmitted to neighboring fibers along the length of the band, and c) some myocardial fibers conduct stimuli more quickly; they are found in the specific conduction tissue distributed by the subendocardium and directly connected to the atrioventricular conduction system. This latter property provides the foundation for mapping the start of cardiac electrical activation, which appears to contradict the sequential contraction along the myocardial band described above.
Fiber direction and grouping in the band are essential. The spatial direction of contraction defined by the band architecture itself influence working effectiveness and the complexity of its interpretation. Take, for example, the linear contraction of a skeletal muscle in which the only parameter is contraction, because its thickening determines the function. Here, thickness reduces the cavity, which makes it an active-effective parameter. Since the start and end points of the band are fixed, the reduction in ventricular cavity volume is substantiated by increased wall thickness, as well as by the effect exerted by the contraction of the descending segment of the apical loop. Because the band is made up of multiple, consecutive contractile segments arranged on top of and underneath each other, the direction of contraction within the helix varies for each segment throughout the process, and neighbouring segments—especially contracted ones—provide the support points for the segments that start to contract.
The band's helical arrangement in itself provides a torsional movement, with the 2 helices sliding over each other along the longitudinal axis, which produces a screw motion and distances the apex from the ventricular base.
In short, successive activation and contraction of the right and left segments of the basal loop narrow (Fig. 1, points 1 and 2) and secure the base of the left ventricle; the contraction of the descending segment of the apical loop (Fig. 1, point 3) screws the base closer to the apex, shortens the length of the ventricular cavity and ejects the blood toward the outlet chamber of the left ventricle. Early and independent contraction of the left ventricular subendocardium generates an apical cavity with a specific morphology that is sufficiently rigid to facilitate the bolus effect. Probably, by the time the ascending segment of the apical loop (Fig. 1, point 4) contracts and diastole starts, the subendocardium is no longer contracted.
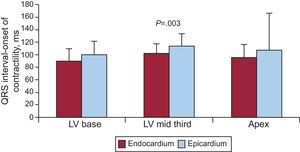
This would explain the apparent contradiction between the electrical activation maps and heart function according to Torrent Guasp's theory. It is based on the measurements taken when comparing contraction times in dog subendocardium and epicardium (Fig. 2). Other authors have also described this phenomenon of early endocardial contraction.14,15
Experiments in open-chest pigs (n=8). The graph shows mean (standard deviation) values for the times of onset of contraction at 3 epicardial points selected at random, and their corresponding points on the endocardium. In all cases, the contraction occurred significantly earlier at the endocardial points (own unpublished data). LV, left ventricle.
It is accepted that ventricular diastole is an active process linked to muscle contraction.7,9 The contraction of the ascending segment of the apical loop unscrews the base, separates it from the apex, and produces a suction effect that creates negative pressure in the ventricular chamber and initiates transmitral flow. Several authors have contributed their experiences to demonstrate this suction effect16 and to show how this disappears when the last segment of the myocardial band is blocked.8
CONCLUSIONSHeart function is highly complex. To understand it, new perspectives have been created by dissecting the heart wall along the myocardial band. This issue includes images from diffusion tensor MR imaging that reproduce the band described by Torrent Guasp, arranged and folded in its anatomical position. The real existence of this band has increased our understanding of cardiac function, and yet with every answer that is obtained, many more questions arise. Thus, if contraction is initiated in the subpulmonary area (in addition to the subendocardium in the Purkinje network), how does electrical activation arrive there? Further research is needed, with new methods and approaches in order to break with preconceived notions.
CONFLICTS OF INTERESTNone declared.
The authors are grateful to Dr. Alicia Maceira González for the information she contributed on diffusion tensor MR imaging.