Marfan syndrome is an inherited disease of the connective tissue. Aortic rupture and dissection are the main causes of mortality in these patients. Recent trials have indicated the use of losartan (a transforming growth factor beta inhibitor) in these patients prevents aortic root enlargement. The aim of our clinical trial is to assess the efficacy and safety of losartan versus atenolol in the prevention of progressive dilation of the aorta in patients with Marfan syndrome.
MethodsThis is a phase III clinical trial conducted in two institutions. A total of 150 subjects diagnosed with Marfan syndrome, aged between 5 and 60 years, of both sexes, and who meet the Ghent diagnostic criteria will be included in the study, with 75 patients per treatment group. It will be a randomized, double blind trial with parallel assignment to atenolol versus losartan (50mg per day in patients below 50kg and 100mg per day in patients over 50kg). Both growth and distensibility of the aorta will be assessed with echocardiography and magnetic resonance. Follow-up will be 3 years.
ConclusionsEfficacy of losartan versus atenolol in the prevention of progressive dilation of the aorta, improved aortic distensibility, and prevention of adverse events (aortic dissection or rupture, cardiovascular surgery, or death) will be assessed in this study. It will also show the possible treatment benefits at different age ranges and with relation to the initial level of aortic root dilation.
Keywords
.
INTRODUCTIONMarfan syndrome (MS) is an autosomal dominant disease that affects the connective tissue. The incidence of this disease is estimated at 1 per 3000 to 5000 births, with no differences by race. It is considered to be one of the most common “rare diseases”.1, 2, 3 The genetic defect is due to a mutation in the gene that codes for fibrillin, which is a necessary protein for the proper assembly of collagen fibers. According to this model, the defective microfibrils that make up the extracellular matrix create “weak” connective tissue, which would cause the symptoms suffered by these patients. However, this structural theory does not completely explain the wide range of manifestations caused by this condition, such as bone overgrowth, osteopenia, reduced muscle mass, and craniofacial anomalies.3 Several recent studies have researched the regulatory function of microfibrils in the extracellular matrix, revealing a different pathogenic mechanism. Tests with animal models have shown that many pulmonary, cardiovascular, and skeletal manifestations are due to overactivation of the transforming growth factor beta (TGF-β), which in turn induces fibrosis, acts as a proinflammatory agent, and activates certain metalloproteinases.4, 5 In the opinion of some authors, the combination of structural alterations in the extracellular matrix and dysregulation of homeostasis driven by overexpression of TGF-β would explain the majority of symptoms observed in these patients.6, 7
This disease causes a wide spectrum of clinical manifestations. The musculoskeletal and cardiovascular system can be affected, as well as skin and eyes, among others. Survival is primarily conditioned by the severity of cardiovascular damage, and complications of the aorta (dissection or rupture) are the most frequent causes of death.8 Approximately 90% of MS patients have some type of cardiovascular complication over the course of their lives, such as aortic root surgery, aortic dissection, or mitral valve surgery.
Several different studies9, 10, 11, 12 have pointed towards the benefits of beta-blocker (BB) treatment in MS patients, which decreases the growth rate of the aorta and the number of complications. Other studies have analyzed the effects of antihypertensive drugs such as calcium antagonists and angiotensin converting enzyme inhibitors, showing that these also have similar or even greater effects than BBs.13, 14
Habashi et al.6 have shown, using rats with fibrillin-1 mutations, that losartan (angiotensin II type 1 [AT1] receptor antagonist) can prevent the progression of aortic dilation and other manifestations of MS. The structure of the aortic wall progressively deteriorated in untreated rats and those that received propranolol, whereas those treated with losartan had aortas that were indistinguishable from those of healthy rats after 6 months treatment. Losartan also improved other noncardiovascular conditions related to the decrease in TGF-β: blood pressure (BP) and heart rate were reduced in rats treated with BBs or losartan. These results reveal that the effect of losartan is conveyed primarily by antagonizing the action of TGF-β. Based on these advances, a research study is being sponsored by the National Heart, Lung, and Blood Institute (ClinicalTrials.gov) in 20 hospitals in the United States, Canada, and Europe comparing atenolol and losartan in reducing the rate of aortic dilation in children and young adults with MS and dilations greater than 3 standard deviations above the mean value (Z-score>3).
The aim of this study will be to compare the efficacy and safety of losartan versus atenolol for preventing aortic dilation in MS patients.
METHOD DesignA phase IIIb, randomized and double blinded study with parallel assignment to one of the 2 treatment groups will be coordinated between 2 institutions (Hospital Universitario 12 de Octubre, Madrid, and Hospital Universitario Vall d’Hebron, Barcelona), in order to evaluate the efficacy and safety of losartan and atenolol in the prevention of progressive aortic dilation in MS patients.
Study PopulationThe study will involve patients aged between 5 and 60 years, which complies with the Ghent criteria for diagnosing MS.15 We will also include cases of minimally dilated aortas. This study design will elucidate the behavior and activity of these drugs in the initial stages of aortic dilation. Along this line of research, Shores et al.9 have already shown that preventive treatment with BBs has a greater effect when the aorta is less dilated. Although children are theoretically the patient population that would receive the greatest benefit from a treatment that slows down the rate of growth of the aorta, it is also important to analyze its effects in the adult population. Patients older than 25 years were not included in the previously mentioned multicenter study; therefore, analyzing the effect in these patients’ aortas would be interesting, especially when this is the population in which the greatest number of complications is produced (aortic dissection and rupture). The inclusion and exclusion criteria for our study are presented in Table 1. Based on estimates made from previous studies, an effective treatment for aortic dilation would reduce the number of patients in which the growth of the aortic diameter is greater than 1mm/year, thus a total growth >3mm in 3 years. Magnetic resonance imaging (MRI) is the most reliable and reproducible technique for measuring aortic diameter, as it can detect changes as small as 1mm. We have estimated that the sample size necessary for detecting these changes with a discriminative power of 0.8 and a 95% confidence level is 60 patients per treatment. Assuming patient drop-out rates of 7% to 10%, the sample size necessary for our study would be 75 patients per treatment group. Our predicted study sample will be 150 patients (100 in the Hospital 12 de Octubre and 50 in the Hospital Vall d’Hebron). This distribution is based on the trend of a greater patient volume observed in the Marfan Unit in the Hospital 12 de Octubre, which is the first specialized unit for this disease in Spain.
Table 1. Inclusion and Exclusion Criteria.
| Inclusion criteria | Age between 5 and 60 years |
| Maximum aortic diameter <45 mm | |
| Women of child-bearing age, negative gonadotropin results from pregnancy test | |
| Exclusion criteria | |
| General | In women: pregnancy, desire for pregnancy, suspicion of pregnancy, or lactation |
| Current participation in another clinical trial or having received either of the research drugs within one month before inclusion in the study | |
| Incapacity to comply with study protocol | |
| Disease characteristics | Previous surgical background: surgery to the heart or any section of the aorta |
| Functional class III-IV | |
| Maximum aortic diameter >45 mm | |
| Moderate or severe aortic valve involvement | |
| Background | History of or current respiratory, liver, kidney (creatinine clearance rate <30 mL/min), gastrointestinal, hematic, or endocrine failure, or any other clinical situation that the researcher feels may affect the evaluation of results |
| Background of aortic dissection | |
| History of or current neurological disease (especially convulsions, dementia, etc.) | |
| History of or current excessive consumption of alcohol and/or toxic substances | |
| Clinically uncontrolled depression | |
| Treatment | Any need for a different drug associated with antihypertensive effects |
| Hypersensitivity, intolerance, or contraindications for any of the study drugs |
We will establish an a priori randomized system for assigning each patient to a treatment group. We will also prepare two envelopes for each patient containing the patient number, his/her assignment to either treatment A or treatment B, and the A/B assignment for each treatment group. One of these envelopes will be given to the clinician in charge of adjusting medication dosages, and the other will remain sealed until the data collection phase of the study has terminated, or it must be opened for some other reason. All envelopes, opened or not, will be compiled at the end of the study. All patients that comply with inclusion criteria and have no exclusion criteria will be randomly assigned to one of the treatment groups on day 0 of the study. Treatments will be assigned to each patient based on the chronological order in which they are included in the study at each hospital. The medication box for each patient will be marked with the treatment group and a description of the treatment assigned.
All patients that were previously receiving one of the drugs (losartan or atenolol) will go through a 2-week “clearing period.” Once the inclusion criteria have been confirmed and the patient has given informed consent, the pharmacy department will randomly assign the treatment to each patient. Only the pharmacy department will know which drug treatment is being given to each patient. Losartan tablets will be administered as the experimental drug. The initial dose will be 25mg/day for patients >50kg, and 12.5mg/day for patients <50kg. After 15 days, depending on clinical tolerance and Holter BP measurements, dosage will be raised to 50mg/day and 25mg/day, respectively. Based on patient tolerance, dosage will be doubled after 1 year for all patients. Atenolol will be administered orally as the control drug, with the same dosage as losartan. If symptoms compatible with hypotension appear, and if systolic BP falls below 100mmHg, dosage will be reduced. Both drugs will be administered during the entire 3 years of the study. In the case of patient intolerance to minimal dosages, treatment will be suspended.
Study Variables and Evaluation of Patient ResponseThe primary variable to be studied is the progression of aortic dilation. This is based on the fact that MS patients suffer progressive aortic dilation, which is the most commonly seen cardiovascular manifestation of the disease. We will measure aortic diameter at different segments of the aorta (aortic ring, sinuses of Valsalva, sinotubular junction, ascending aorta, aortic arch, thoracic aorta, and abdominal aorta) using MRI and echocardiograms.
The secondary variables to be measured are: a) adverse events during follow-up (aortic dissection or rupture, need for surgery to the aorta or heart, and death); b) adverse effects of losartan and atenolol; c) distensibility of the aortic wall as measured using MRI and echocardiogram; d) echocardiographic examination of the aortic valve, mitral valve, ventricular functioning, and the size of the left ventricle; and e) evolution of dural ectasia using MRI in patients younger than 12 years. Furthermore, these secondary variables will be used to determine the safety of administering losartan as opposed to atenolol.
The timeline for clinical evaluations and imaging tests is summarized in Table 2.
Table 2. Patient Evaluation Timeline.
| Initial | 6 months | 12 months | 24 months | 36 months | |
| Consent | X | ||||
| Demographic data, weight, height, BMI, and physical exam | X | ||||
| Randomization | X | ||||
| Clinical visit | X | X | X | X | X |
| BP measurement | X | X | X | X | X |
| Laboratory analyses | X | X | X | X | |
| Doppler-ultrasound | X | X | X | X | X |
| Cardiac MRI | X | X | |||
| Adverse events | X | X | X | X |
BMI, body mass index; BP, blood pressure; MRI, magnetic resonance imaging.
After 15 days from the start of the treatment, we will perform telephone interviews in order to evaluate the possible adverse effects and need to measure Holter BP in the case of suspected symptoms secondary to hypotension.
Echocardiograms and MRIs will be performed by the same two expert observers for each imaging test at the two institutions. Each observer will independently assess each exam without knowing the patient's details. Before commencing the study, each observer will perform several imaging tests in order to ensure proper methodology. Each center will be charged with all the measurements for one of the two imaging techniques throughout the study. The measurements will be performed by the same person each time, who will not know the clinical information of the patients or the results from the other imaging test. Inter- and intraobserver variability will also be estimated for each of the variables analyzed.
Echocardiogram1. Morphological analysis, specially designed to diagnose mitral valve prolapse and dilation of the pulmonary artery: Measurements of the aorta will be obtained using M-mode and bidimensional ultrasound. These techniques will be used to measure the internal diameter of the vessel in the aortic ring, sinuses of Valsalva, sinotubular junction, tubular ascending aorta, descending thoracic aorta, and abdominal aorta.
2. Biomechanical analysis of the aorta:
– Pulse wave velocity (PWV) will be calculated using the formula PWV=ΔD/Δt. ΔD will be the distance between the two levels of the aorta measured using the bidimensional image and the externally measured suprasternal-abdominal distance. Δt will be the pulse transit time between the 2 levels of the aorta obtained from the suprasternal and subcostal images. To obtain the values of Δt, we will use pulsed Doppler ultrasound at each level of the aorta to measure the time from a fixed baseline QRS to onset of systolic flow.
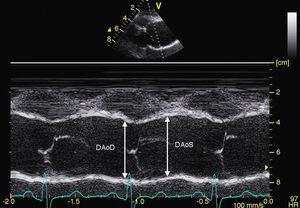
– For the analysis of aortic stiffness: systolic and diastolic aortic diameters will be measured using M-mode ultrasound in the sinuses of Valsalva, the ascending aorta, and the abdominal aorta (Figure 1). We will use these measurements to calculate the following rates of aortic elasticity: strain=[(Ø systolic Ao–Ø diastolic Ao) / Ø diastolic Ao]×100, and distensibility=[2×(Ø systolic Ao–Ø diastolic Ao]×100, and distensibility=[2×(Ø systolic Ao–Ø diastolic Ao) / (diastolic Ao×PP)] (cm2×din–1×10–6). Brachial pulse pressure (PP) will be obtained using sphygmomanometry.
Figure 1. Measurement of aortic diameters using M-mode ultrasound in the aortic sinuses of Valsalva in systolic and diastolic phases.DAoD, diameter of the aorta in diastole; DAoS, diameter of the aorta in systole.
We will obtain the following sequences with electrocardiographic synchronization:
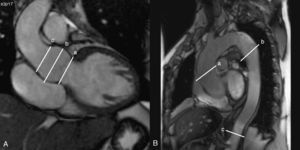
1. Steady-state free precession imaging will be evaluated using oblique sagittal and coronal cuts of the aorta to be used as an anatomical reference for making transverse cuts at different levels of the aorta: the aortic ring, ascending aorta (at the pulmonary artery bifurcation), descending aorta (distal to the aortic arch, and at the height of the pulmonary artery bifurcation), and the abdominal aorta distal to the diaphragm. These anatomical reference points will be used in follow-up analyses. We will measure the diameter of the aortic ring, the ascending aorta, the descending aorta, and the abdominal aorta. All diameters will be measured in two orthogonal planes and divided by body surface area in order to obtain index values (Figure 2). We will also measure maximum (systolic) and minimum (diastolic) aortic area at each level. Aortic distensibility will be calculated using the equation: D=(Amax-Amin)/(Amin×PP), where D is distensibility (in mmHg−1), Amax is maximum aortic area (systolic, in mm2), Amin is minimum aortic area (diastolic, in mm2), and PP is brachial pulse pressure measured by sphygmomanometry. We will calculate PP as the difference in mmHg between systolic pressure (SBP) and diastolic pressure (DBP). We will also calculate mean BP: DBP+1/3(PP).
Figure 2. A: coronal slice of the aortic root in which the following can be observed: a) aortic ring; b) sinuses of Valsalva; and c) sinotubular junction. B: oblique sagittal slice of the aorta in which the following are observed: a) ascending aorta; b) descending aorta, and c) abdominal aorta.
2. Velocity-coded phase-contrast sequences: we will use the same previously mentioned levels of the aorta that were used to calculate PWV. The difference in time between the arrival of the flow waves will be measured at each level. We will also measure the distance between each level and calculate PWV using the same equation used for the echocardiogram results: PWV=ΔD/Δt.
We will use the QFlow software package, version 5.1, as our flow analysis work platform (Medis, Leiden, Netherlands).
Statistical AnalysisResults will be expressed as mean (standard deviation) of individual values or as percentages. Means between the two groups will be compared using two-tailed Student's t-test, and the Mann-Whitney U test will be used for nonparametric variables.
Patient risk will be estimated using the incidence of adverse events. Cumulative incidence tends to underestimate risk when the follow-up period is short, and event-free survival is preferable in this case. As such, a survival analysis will be carried out using Kaplan-Meier tables for death, aortic dissection, rupture of the aorta, need for cardiovascular surgery, and aortic regurgitation.
Changes in aortic diameter are the consequence of two different processes: the natural biological growth of the patient, particularly in children and young adults, and dilation of the aorta secondary to collagen defects due to the disease. In order to determine these two variables, we will calculate an aortic quotient by dividing the diameter of the aorta measured using echocardiogram by the expected diameter based on body surface area and the age and sex of the patient. All analyses will be performed using SPSS® software (Statistical Package for Social Sciences).
EthicsThis study has been authorized by the Ministerio de Sanidad y Consumo (Spanish Ministry of Health and Consumption), and has received positive reports from the ethics committees at both hospitals, as well as from the Agencia Española del Medicamento (Spanish Drug Authority). This study will be carried out in accordance with the ethical principles set out by the Helsinki Declaration, and complies with good clinical practice and the applicable regulatory guidelines. The researchers have no conflicts of interest, and financing for the project comes from a research grant obtained from the Fondo de Investigaciones Sanitarias del Instituto de Salud Carlos III (Carlos III Institute Health Research Fund), which is also covering the cost of medications for the study.
DISCUSSIONThe survival of MS patients is primarily conditioned by aortic complications (aortic dissection and rupture). MS patients must unconditionally receive drug treatment in order to prevent cardiovascular complications, which cause 90% of the deaths produced by this disease. In spite of the fact that prophylactic use of BBs is accepted as normal practice for MS patients in the majority of hospitals, studies with other antihypertensive drugs have also shown beneficial effects that even surpass the results from BBs. The study by Yetman et al.13 showed that enalapril produced better results than propranolol and atenolol, improved aortic stiffness and slowed down aortic growth. A metaanalysis published in 2006 by the Mount Sinai group16 even concluded that there was no evidence that BBs provided any benefits for MS patients.
Recently, the clinical usefulness of treatment with losartan was shown in MS children. Brooke et al.17 studied 18 MS children and dilation of the aortic root after receiving other treatments that had not been effective at stopping or even slowing down the progressive growth of the aorta. They administered losartan to 17 children and irbesartan to 1 child, with a follow-up period of 12 to 47 months. In spite of the small and very specific patient sample, they observed that the use of both drugs significantly reduced the progression of aortic root growth. Losartan is an oral angiotensin II AT1 receptor antagonist.18 In addition to its antihypertensive effect, AT1 receptor blockade induces a decrease in the plasma concentrations of TGF-β, the genetically controlled cellular response, and the intracellular mediators of the TGF-β signaling cascade, such as Smad 2.19, 20, 21 Furthermore, it does not interfere with the AT2 receptor which, as opposed to AT1, has a beneficial intracellular effect, acting as an antiproliferative and anti-inflammatory agent. It also contributes to proper homeostasis in the aortic wall.22
Although 6 different randomized prospective clinical trials have been performed (Table 3) that analyze the usefulness of losartan compared to BBs (atenolol or nebivolol), or losartan versus previous treatment along with placebos, our study can provide information regarding specific details that would be difficult to obtain from other studies. We include patients aged 5 years to 60 years, and the only aortic dilation criteria was that it measure <45mm. As such, we expect to obtain comparative information regarding the benefits of each drug with respect to age and the initial level of aortic dilation. In addition, we will compare the echocardiographic results at increasing dosages of atenolol and losartan during the first year, and the maximum tolerable doses in the following 2 years. The reference method for evaluating changes in aortic diameter and biomechanical properties of the patient will be an MRI obtained immediately after the start of treatment and after 3 years of treatment, given its excellent reproducibility in measurements.
Table 3. Main Studies that Compare Beta-Blockers With Losartan for Marfan Syndrome.
| Institution | Royal Brompton Harefield NHS Foundation | Hospital Bichat Paris | Ghent Hospital | Boston Children's Hospital, Pediatric Heart Network | Academic Medical Center Amsterdam | Policlinico S. Matteo Hospital |
| Country | United Kingdom | France | Belgium | United States, Canada, and Belgium | Netherlands | Italy |
| Design | Multicenter | Multicenter | Multicenter | |||
| No. cases | 490 | 300 | 174 | 604 | 330 | 291 |
| Age range (years) | 4-40 | >10 | >10 | 0.5-25 | Adult age | 1-55 |
| Drug | Irbesartan | Losartan | + losartan | Losartan | + losartan | Losartan |
| Daily dosage | 300 mg | 50mg if <50 kg; 100mg if >50 kg | 50mg if <50 kg; 100mg if >50 kg | Atenolol 0.5-4 mg/kg; losartan 0.3-1.4 mg/kg | Losartan 100 mg | Nebivolol 0.16 mg/kg or 10 mg/day for adults; losartan 1.6 mg/kg or 100mg if >50 kg |
| Additional treatment | Irbesartan (75mg, 1 month) | No | BB | No | BB | No |
| Control drug | Placebo | Placebo | + placebo | Atenolol | + placebo | Nebivolol; losartan+nebivolol |
| Start date | September 2010 | September 2008 | June 2009 | January 2007 | February 2008 | July 2008 |
| Follow-up (months) | 60 | 36 | 36 | 36 | 36 | 48 |
| Objectives | Yearly change in aortic root diameter (ultrasound) | Aortic diameter change in the sinuses of Valsalva (ultrasound) | Diameter of the aortic root adjusted to body surface area, Z-score (ultrasound) | Change in the diameter of the aortic root adjusted to body surface area, Z-score (ultrasound) | Change in the aortic diameter in its entirety (cardiac MRI/CT) | Aortic root diameter adjusted for age and body surface area |
BB, beta-blocker; CT, computerized tomography; MRI, magnetic resonance imaging.
A 3-year follow-up period is probably too short to evaluate the differences in major clinical results between the 2 groups. As such, the primary objective of this study is to use imaging tests to assess the initial development of each patient, rather than to reach a long-term clinical conclusion. We have used very robust imaging diagnostic techniques for detecting biomechanical parameters (aortic rigidity and stiffness) that could have future clinical repercussions. The absence of a control group is due to ethical considerations, as it is already well established that some type of pharmacological treatment is necessary to prevent cardiovascular complications in these patients. Even so, the absence of a placebo treatment implies that we will not be able to evaluate the true efficacy of each drug independently, but rather a comparison between them. The inclusion of both children and adults may be a limitation to the study if the total number of patients does not allow for studying the different effects of both drugs in each subgroup.
CONCLUSIONSThis randomized clinical trial will compare the response to treatment with losartan versus atenolol in MS patients with a wide range of ages and minimally dilated aortas. We will also evaluate the possible adverse effects of and tolerance to losartan and atenolol in this population.
CONFLICTS OF INTERESTNone declared.
Received 3 October 2010
Accepted 13 February 2011
Corresponding author: Servicio de Cirugía Cardiaca, Hospital Universitario 12 de Octubre, Avda. de Andalucía s/n, 28045 Madrid, Spain. apforteza@yahoo.es