Keywords
INTRODUCTION
Aneurysmal conditions of the descending thoracic aorta represent a potentially life-threatening situation with a risk of rupture depending on diameter.1 Surgical resection and interposition of vascular prostheses have long been considered the standard of care despite substantial risk of adverse events and complications from surgical trauma.2 Regardless of recent strides to improve technique and management, operative mortality, and morbidity remained high. As a consequence of demographic changes in the Western world, the population is aging and associated with a variety of comorbidities portending an inherent risk and explaining in part the sobering surgical outcomes with perioperative complications contributing to prolonged hospitalization and high costs.3 As a revolutionary alternative, the concept of an endoluminal stent-graft in patients with thoracic aortic disease has emerged a decade ago propelled by the desire to avoid surgical risks by use of a nonsurgical approach and to induce reconstructive remodelling of the diseased aorta by initiating a natural healing process through exclusion and depressurization of the aneurysmal sac.4-6 Although initial reports on the endovascular stent-graft strategy were encouraging in various pathologies,3,7-9 randomized data are still limited, and smouldering critique has never been fully extinguished because of lacking long-term follow-up data.
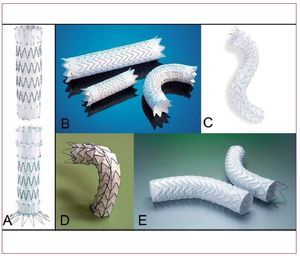
Early clinical experiences with stent-grafting of the thoracic aorta were based on the use of homemade devices that were rigid and required large delivery systems.4 To date, several companies obtained approval for commercial release of their thoracic endografts in the U.S. and Western Europe, and it is likely that other devices will follow them in the marketplace.10-13 Although each device has unique features, all employ the same basic structural design (Figure 1). Generally, endoprotheses are composed of a stent (nitinol or stainless steel) covered with fabric (polyester or PTFE); different designs are available to facilitate endoluminal fixation (bare/covered springs or barbs). The selection of patients on the basis of favourable anatomy and pathology for a specific endovascular device is key to the success of the procedure. Not all patients have lesions amenable to endovascular repair, and thoracic endografting is technically challenging, requiring dedicated facilities, and experienced specialists. Conversely, shortcomings of specific endovascular devices including device collapse, migration, or unprecise launching are not fully resolved.14 This article reviews current indications and advancing fields of endovascular repair in the thoracic aorta.
Figure 1. A selection of thoracic stent-grafts currently available for endoluminal repair. Zenith TX2 by Cook Medical (A); TAG by GORE (B); Valiant by Medtronic AVE (C) ; Relay Thoracic Stent-Graft by Bolton Medical (D); EndoFit by LeMaitre Vascular (E).
ENDOVASCULAR RECONSTRUCTION OF THORACIC AORTIC DISSECTION
Stent-Grafting as an Emerging Option in Type B Dissection
The optimal treatment strategy for patients with aortic dissection confined to the descending aorta (Stanford type B) remains a matter of debate.15 Despite continuous efforts for improvement, surgical resection of dissection is still associated with operative mortality ranging between 0% and 27% in elective cases, and exceeding 50% in complicated dissection under emergency conditions.16
Given such grim outlook with open surgery, there is consensus that patients with type B aortic dissection should primarily be treated medically with tight blood pressure control, while reserving endovascular surgery for evolving complications (eg, recurrent pain, progressive false lumen expansion, malperfusion, or imminent rupture).17 In a recently published series of 384 patients with acute type B dissections from the International Registry of Aortic Dissection (IRAD), 73% were managed medically with an in-hospital mortality of 10%.18
However, even without complications in acute stage, the long-term prognosis of type B dissection was sobering with a reported 3 year mortality of 20%-40% despite optimal medical and surgical therapy.19
In 1999, the concept of endovascular stent-graft implantation was introduced as a novel treatment option propelled by the idea to seal the proximal entry tear, remodel the aorta, and avoid risk of open surgery.20,21
This rationale was originally based on the clinical observation that patients with spontaneous thrombosis of the false lumen have a better long-term prognosis.22
Conversely, perfusion of the false lumen has been identified as an independent predictor of progressive aortic enlargement and adverse long-term outcome.23
Several single-centre reports and multinational registries have corroborated technical feasibility and clinical safety of endoluminal thoracic aortic reconstruction in type B dissection, but final data from randomized trials are not available yet.7-9
The Mechanics of Endovascular Repair in Aortic Dissection
The natural course of aortic dissection is characterized by a continued false lumen expansion carrying the risk for late rupture.24 The most effective method to exclude an enlarging false lumen in type B dissection is to seal the proximal entry tears with a customized stent-graft.20,21
Depressurization and shrinking of the false lumen is the most beneficial result to be gained, ideally followed by complete thrombosis of the false lumen and remodeling of the entire dissected aorta.25,26 In scenarios with dynamic true lumen collapse, malperfusion syndrome may also be corrected by single thoracic endografting27-29; in selected patients distal bare-stent extension (PETTICOAT-concept) may potentially enhance the remodeling process by enlarging the true lumen and re-establishing distal blood flow. (Figure 2).30 Similar to previously accepted indications for surgical intervention, scenarios such as intractable pain, rapidly expanding false lumen, diameter over 55 mm and signs of imminent rupture or distal malperfusion are now accepted indications for stent-graft placement in type B dissection.31-34 Preliminary data suggest that endovascular repair is superior to open surgery in complicated cases of type B dissection with respect to in-hospital morbidity and earl mortality.35,36 Paraplegia generally appears to be a rare phenomenon (0.8%), but is known to be associated with extensive coverage of the aorta >20 cm. and use of multiple stent-grafts.7-9
Figure 2. Endovascular treatment of acute type B dissection presenting with peripheral malperfusion (A). The composite of images depicts the PETTICOAT concept as an adjunctive distal bare-stent extension after deployment of a proximal thoracic stent-graft. Notably, occlusion of the proximal entry tear was followed by thoracic false lumen thrombosis (C, D). The metal scaffolding extension prevents true lumen collapse and ensures normalized distal run-off (B).
Results of short-term follow-up are excellent with a 1-year survival rate of >90%; tears can be readapted and aortic diameters generally decrease with complete thrombosis of the false lumen. This suggests that stent-graft placement may facilitate healing of the dissection, sometimes of the entire aorta, including abdominal segments (Figure 3). However, primary endoleaks and late reperfusion of the false lumen have been observed occasionally underlining the need for stringent follow-up imaging and additional stent-graft placement in some patients.37-39 To clarify the role of prophylactic endovascular repair, final results from the randomized INSTEAD trial are awaited comparing the outcome of treating uncomplicated Type B dissection with a Talent stent-graft in addition to best medical treatment versus best medical treatment alone,40 while interim analysis has not provided a survival benefit of stent-graft therapy within a year. Stent-graft treatment in chronic type B dissection apparently differs from acute type B pathology based on increased stiffness of the dissecting lamella and a continued false lumen expansion. Endograft deployment in chronic dissection does not necessarily focus on expansion of the true lumen, but aims to depressurize the false lumen by promoting progressive thrombosis. This remodeling process is often complicated by the rigidity of the dissecting lamella, as reflected by the higher amount of procedural failure in chronic dissection.7 Furthermore, as the individual case selection is important, some comorbidities such as connective tissue disorders and general state of health need to be considered. For instance, in Marfan patients endovascular strategies may only be justified to bridge to definite surgical repair, but failed to impact on early outcomes.41,42 Moreover, general state of health prior to endovascular therapy has demonstrated to influence postprocedural outcomes.43
Figure 3. Stent-graft induced aortic remodelling in acute type B dissection. Note the communications between the true and false lumen at the thoracic and abdominal level (A). After stent-graft placement across the proximal thoracic entry, the entire aorta including the abdominal segment is reconstructed (B). With time "healing" of the dissected aortic wall occurs by means of progressive shrinkage of the thrombosed false lumen (C).
Endovascular Approach to the Proximal Aorta
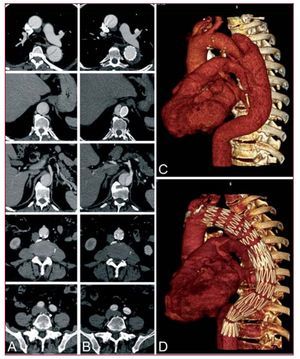
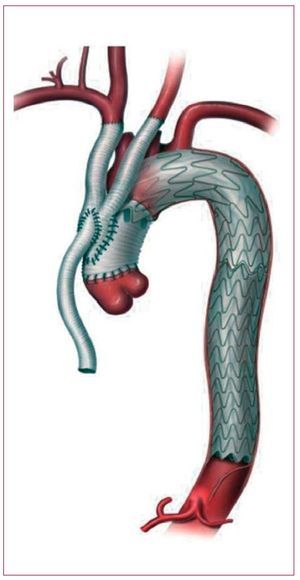
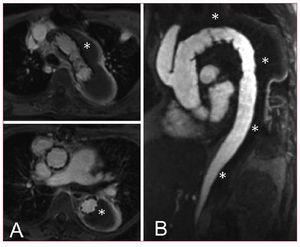
Two-third of patients hospitalized for aortic dissection is diagnosed with Stanford type A dissection characterized by an entry in the ascending aorta. Distal involvement is frequently observed with the dissection lamella propagating into both aortic arch and descending aorta in over 70%. Acute type A dissection is an emergency and requires swift surgical replacement of the ascending aorta; only in selected cases exclusive endovascular approach to the proximal aorta may be an option (Figure 4). According to IRAD, 92% of patients qualify for replacement of the ascending aorta; of those 23% also required partial arch and 12% required total arch replacement. Overall, 91% of patients underwent repair utilizing cardiopulmonary bypass in hypothermic circulatory arrest, while 52% were subjected to antegrade cerebral perfusion. In addition to 25% in-hospital mortality among surgically treated patients, a patent false lumen remains in the aortic arch in up to 75% of cases requiring distal reoperations in about a quarter of surviving patients.44 Considering such pessimistic outlook, type A dissection deserves reconsideration especially with emerging endovascular technology. One approach could be a staged hybrid procedure with initial replacement of the ascending aorta and simultaneous aorta-inominate artery bypass without hypothermic circulatory arrest followed by a staged left carotid bypass and transfemoral endograft to exclude the perfused distal false lumen.45 The objective of this approach is to avoid surgery on the arch with its inherent problems, but rather complete the repair with a retrograde endograft. This hybrid endovascular approach not only minimizes the risk of each surgical step, but also enables diligent evaluation of distal false lumen prior to stent-graft placement. In this setting, even single stage techniques combining open ascending tube graft insertion with simultaneous great vessel transposition and antegrade deployment of an endoluminal graft across the arch and the descending aorta is feasible (Figure 5).46 This demanding and time consuming approach requires the skills of both cardiac and endovascular surgeons and intraoperative fluoroscopy, but lacks the precision of a staged procedure.47 Moreover, there is some resistance to one-stage endoluminal grafting in acute type A dissection as physicians are aware of fragile tissue and friable dissecting lamella likely to be injured or perforated by antegrade positioning of a stent-graft under non-circulating conditions. At present there are no endografts designed especially for use in the ascending aorta, nor for correcting dissections. Such challenges will certainly be addressed by customized stent-graft technology in the near future. Nevertheless, the concept of one-step hybrid repair with an antegrade stent-graft delivery might become part of the therapeutic armamentarium for complex type A dissections with life-threatening distal malperfusion, while a staged repair with retrograde stent-delivery appears to be a better option in stable situations (Figure 6).
Figure 4. Nonsurgical exclusion of a localized tear in the ascending aorta. A short customized endograft was retrogradly advanced from the femoral artery to cover the entry of the lesion.
Figure 5. Illustration of single-stage repair in acute type A dissection combining an open ascending tube graft with simultaneous great vessel transposition and antegrade deployment of an endoluminal graft across the arch and the descending thoracic aorta.
Figure. 6. Staged hybrid approach for type A dissection with initial open ascending tube graft insertion and simultaneous head vessel debranching. At second stage, the distal false lumen was excluded by means of transfemoral stent-graft deployment across the aortic arch.
ENDOLUMINAL TREATMENT FOR DESCENDING THORACIC ANEURYSMS
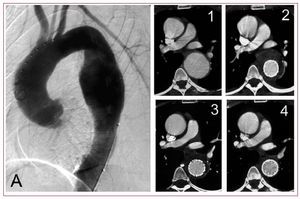
Degenerative aneurysms may involve one or more thoracic aortic segments and are classified accordingly. Sixty percent of thoracic aneurysmsaffect the ascending aorta, 40% are exclusively confined to the descending aorta, while another 10% involve the arch or extent into abdominal segments, respectively. Aetiology, natural history, and treatment options differ for each of these segments.48 Observational studies revealed an average expansion of 0.1 cm. per year; however, the growth-rate was higher in aneurysms affecting the descending rather than ascending aorta and was accelerated in Marfan-patients. For aneurysms exceeding 6 cm in diameter, the individual risk appears to rise to an annual rupture rate of 7%.49 In this scenario, endovascular stent-graft exclusion offers a promising therapeutic alternative with the advantage of a nonsurgical procedure potentially lowering postoperative morbidity and mortality (Figure 7).4,50-52 Recently, European multicenter-registries reported primary technical success in 80%-90% of cases treated endoluminally for descending thoracic aneurysm.8,9 Major procedure-related neurological complications—including stroke and spinal cord ischemia—occurred in up to 8%.
Figure. 7. Preoperative angiography in a 52 year-old man with a thoracic aortic descending aneurysm (A). Computed tomography scans after successful stent-graft exclusion of the aneurysm demonstrate continued shrinkage of the periprothetic thrombus mass (1-4).
However, compared with standard open surgical repair, endovascular treatment appears to halve perioperative mortality with similar late survival and almost identical rates of reinterventions and ischemic spinal cord complications.53,54 Patients considered suitable for stent-graft placement should have a proximal and distal segment of relatively normal aorta for fixation with satisfactory seal. These regions are often referred to as "landing zones" or "aortic neck" and should ideally encompass more than 20 mm free of aortic wall atheroma or thrombus. Close proximity to the arch vessels may complicate endografting for thoracic aortic aneurysm, necessitating intentional coverage of the left subclavian artery or even prophylactic bypass-surgery in selected cases.55,56 At present, endovascular therapy is best reserved for those individuals with a suitable anatomy or poor surgical candidates.57
Technical refinements and miniaturization of the devices are required before routinely used for any aneurysm.
ENDOVASCULAR OPTIONS FOR INTRAMURAL HAEMATOMA
Approximately 5% of patients admitted to the hospital with suspected acute aortic dissection will be diagnosed as intramural haematoma (IMH).58-60 IMH is characterized by haematoma formation between medial layers of the aortic wall without an associated intimal tear probably caused by spontaneous rupture of the aortic vasa vasorum with propagation of subintimal hemorrhage.61,62 Compared with classic aortic dissection, intramural haematoma is observed more often in descending rather than ascending segments and predominantly occur in elderly patients.63
Malperfusion and pulse deficits are rare, although progression to frank aortic dissection occurs in 16% to 36%. Recently, observational studies demonstrated that a normal aortic diameter in the acute phase is the best predictor of survival with regression to normal morphology in one third of patients.64,65 In a prospective study of 68 consecutive patients with intramural haematoma (12 type A, 56 type B) a maximum diameter over 50 mm and ascending aorta involvement were both predictive of early mortality.66 Similar to classic aortic dissection, early surgical graft repair should be standard treatment of IMH in the ascending aorta. Conversely, asymptomatic patients with involvement of the descending aorta can be monitored closely while on medical treatment,67 with endovascular intervention reserved for patients who develop complications such as persistent pain, penetrating atherosclerotic ulcer, signs of impending rupture, or enlarging aortic diameter.17 Nonsurgical strategies such as endovascular placement of stent-grafts to cover the intramural haematoma have recently been associated with a 30-day mortality ranging from 0% to 16% and thus compare favourably with open surgical repair. On aggregate, IMH in the descending aorta per se is no reason for stent-grafting; however, in cases with progressive complications originating from IMH, endovascular strategies may be considered ranging from entry closure in dissection to exclusion of a local aneurysm or penetrating ulcer.
ENDOGRAFT MANAGEMENT OF PENETRATING ULCER AND PSEUDOANEURYSM FORMATION
The term "penetrating atherosclerotic ulcer" (PAU) describes a condition in which ulceration of an atherosclerotic plaque penetrates the internal elastic lamina and allows haematoma formation within media of the aortic wall.68,69 PAUs are most frequently located in descending thoracic aorta and in elderly patients with a history of hypertension, smoking, and other atherosclerotic disease, eg, pre-existing abdominal or thoracic aortic aneurysm. In one quarter of cases atherosclerotic ulcers may penetrate through the media to form saccular aortic pseudoaneurysm or less often perforate the adventia and cause transmural aortic rupture.40-43 The entity is associated with a variable amount of localized intramural haematoma, but may extend to classic aortic dissection in rare cases.70,71 At present, no definite treatment strategy has been established for managing penetrating atherosclerotic ulcers. Certainly, unstable patients with evidence of contained rupture should undergo urgent repair. Continued or recurrent pain, distal embolization, and progressive dilatation are also indications for surgery. However, it remains unclear if stable patients with PAU should undergo surgery or medical management.72 There is growing optimism that transluminal stent-graft placement may become an alternative to open surgery, as limited aortic disease constitutes an ideal condition for endovascular repair (Figure 8). Numerous case reports and smaller series confirm the short-term safety of stent-graft placement in symptomatic patients,73,74 but long-term efficacy will require careful matching of endovascular repair to understand principles of patient selection and treatment modalities.
Figure 8. Penetrating aortic ulcer diagnosed in an elderly male presenting with acute chest pain (A). The patient was considered to be at high risk for rupture. Emergency application of a thoracic stent-graft enabled complete sealing of this limited aortic lanceration (B).
STENT-GRAFT REPAIR OF TRAUMATIC AORTIC TRANSSECTION
Traumatic aortic rupture commonly occurs as a consequence of rapid deceleration forces, including car and motorcycle collision, fall from height, or blast injuries. It accounts for up to 20% of fatal motor vehicle accidents with prehospital mortality rates between 80% and 90%.75-77
The region subjected to the greatest strain is the isthmus, where the relatively mobile thoracic aorta joins the fixed arch at the site of the ligamentum arteriosum; aortic rupture occurs here in 90% of cases in clinical and pathological series.75,78,79 The lesion may be limited to the intima, but can potentially encompass all aortic layers forming medial laceration, false aneurysm, and periaortic hemorrhage.78 In the vast majority of patients who survived the initial traumatic impact, affection of both the intimal and medial layer results in a localized outpouching of the diseased aortic wall. Despite advances in surgical techniques, mortality of emergent open repair exceeds 15% in current literature, depending on severity of associated traumatic lesions, preoperative shock and use of circulary assistance.77,80,81 A significant reduction of surgical mortality has been accomplished by deliberately delaying open repair in hemodynamically stable patients.82-84
Although this delayed approach is justified by objective data, it has to be taken into account that as many as 4% of patients awaiting surgery die due to aortic rupture within 1 week after traumatic injury.85 With the advent of stent-graft technology this less traumatic therapeutic approach attracts growing interests with no need for thoracotomy, aortic cross-clamping, and cardiopulmonary bypass.86-88 As confirmed by serial imaging, restitution of aortic wall integrity can be achieved in almost all patients treated endoluminally, rendering stent-graft intervention an accepted first-line approach to traumatic aortic conditions (Figure 9). Recently, a comparative meta-analysis reviewed outcomes of 699 patients referred to endovascular or open repair after traumatic aortic transsections. With a technical success rate not different from open repair (96.5% vs 98.5%, P=.58), endovascular therapy was associated with lower periprocedural mortality (7.6% vs 15.2%, P=.0076) and demonstrated an exceptional low incidence of paraplegia (0% vs 5.6%, P<.0001) and stroke (0.85% vs 5.3%, P=.0028).89 Because most injuries occur at the aortic isthmus in younger patients the placement of a sometimes oversized and rigid device into an angulated aortic has raised concerns of iatrogenic injury and failing long-term durability. However, technology is improving and offers newer, more flexible, and smaller devices. At present, standard thoracic stent-grafts are available, allowing their effective application in an emergency setting to prevent exsanguation from traumatic aortic rupture.80,90-93
Figure 9. Traumatic aortic tear (arrow) diagnosed in a young man after motorcycle collision (A). The hemodynamically stable patient was elected for delayed endovascular repair 3 weeks after the initial traumatic impact. Note the complete attachment of the intimal lesion after stent-graft placement (B).
ENDOVASCULAR MANAGEMENT OF AORTOBRONCHIAL AND AORTOESOPHAGEAL FISTULAS
Aortobronchial fistulas (ABF) can be associated with a number of pathologic conditions of the descending thoracic aorta, including degenerative and dissecting aneurysms, para-anastomotic pseudoaneurysms secondary to previous open surgical repair, stent-graft erosion of the aortic wall, and mycotic aneurysms.94-96
Urgent intervention for this life-threatening disorder usually requires resection and graft interposition, but at the costs of high risk for death and paralysis, particularly in the setting of hemodynamically unstable patients. Even today, the operative mortality for traditional open repair of ABF using the clamp-and-sew technique reaches 20%.97 Since 1996 aortic stent-grafts have been increasingly used to manage ABF in patients who are at prohibitive risk for direct surgical repair. A recent meta-analysis demonstrated a cumulative 30-day mortality of only 8.3% with most of the cases reporting successful 1-year survival.98
Aortoesophageal fistula (AEF) is another uncommon, but highly fatal condition, occurring most commonly in association with thoracic aortic aneurysms, foreign body ingestion, esophageal malignancy, and traumatic aortic injury.99 Furthermore, secondary AEF is a well documented sequelae of prosthetic and endovascular repair of aneurysmal aortic disease. The widely accepted treatment modality for AEF is thoracotomy with aortic graft interposition, followed by surgical reconstruction of the oesophagus.100,101 This procedure is associated with a high mortality and morbidity rate due to the poor clinical condition of patients at time of surgery. Aortic stent-graft placement for AEF has emerged as a less invasive therapeutic option for vascular repair in these high-risk patients.102 Today, this is confirmed by numerous case reports demonstrating efficacy to both control aortic bleeding and stabilize patients'condition before initiating further treatment.103-106 Despite promising short-term results it remains unclear whether endovascular aortic stenting in an potentially infected area can be seen as definitive treatment or serves as bridging maneuver to allow delayed aortic surgery in patients with aortobronchial or aortoesophageal fistulas.
PERCUTANEOUS ENDOVASCULAR REPAIR OF ANEURYSMS AFTER COARCTATION SURGERY
Coarctation of the aorta occurs in up to 5% of patients with congenital heart disease potentially leading to cerebrovascular morbidity, stroke, and infarction, thus dramatically limiting life expectancy if left untreated.107
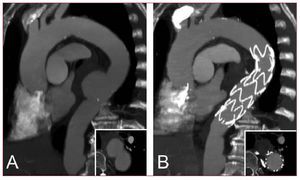
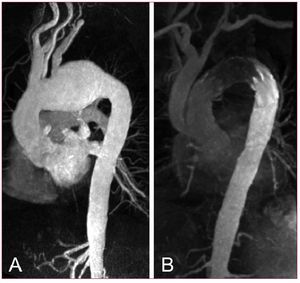
Open surgery used to be the standard of care and surgically treated aortic coarctation was initially considered successful and curative.108-110 It has been recognized, however, that late problems such as re-coarctation, aneurysm formation and potential rupture tend to occur within decades even after successful synthetic patchgraft repair or subclavian flap aortoplasty.111,112 Today, aneurysms forming after previous coarctation surgery are amenable to endoluminal repair as the localized lesion is often accompanied by adequate landing zones ensuring perfect wall apposition of the stent-graft. Since open redo-surgery carries considerable risk, the endoluminal approach might emerge as the treatment of choice. Customized, tapered stent-grafts are now available from various manufactures to manage the potential discrepancies of proximal and distal "aortic neck" diameters in complex aortic lesions (Figure 10). Although preliminary series demonstrated a promising potential of endovascular stent-grafts to avoid thoracic redo-surgery,113-115 the durability of these devices is unproven as long-term results are not available yet.
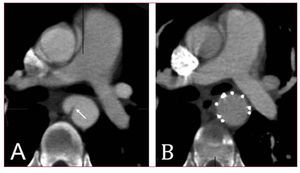
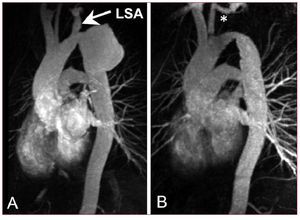
Figure 10. A patient presenting with large pseudoaneurysm 29 years after surgical correction of aortic coarctation (A). The aneurismal defect was excluded with a flexible third-generation endograft after previous transposition of the left subclavian artery (LSA). Narrowed aortic isthmus was left untreated as no significant pressure gradient was measured (B).
INTENTIONAL OCCLUSION OF THE LEFT SUBCLAVIAN ARTERY
Both technical and long-term success of endovascular strategies depend on anatomical conditions for optimal fixation of the endoprothesis, with landing zones 32 cm in length. Close proximity between the left subclavian artery (LSA) origin and the degenerative aneurysm or primary entry tear tear is an important consideration. For this reason, complete coverage of the LSA ostium has to be used to expand the application of endovascular devices to aortic pathologies adjacent to the LSA. A recently published review article analyzed the need for subsequent transposition of the LSA in patients undergoing thoracic aortic stent-graft placement and found that only 4% exhibited early ischemic symptoms of the upper left extremity after intentional LSA occlusion. While 84% of patients were completely asymptomatic at follow-up, only 3% required elective left carotid-tosubclavian bypass due to weakness of the left arm.55 Thus, we do not favour routine prophylactic transposition or bypass-grafting of the LSA recommended by other groups.116-118 Our position is corroborated by the fact that most patients with an ultrasound-documented subclavian steal are asymptomatic.119 In addition, collateral perfusion of the left arm appears sufficient, as has been seen when antegrade flow of the LSA is sacrificed after surgery for aortic arch coarctation.120 Furthermore, auxiliary surgical revascularization procedures add to the overall risk of endovascular aortic reconstruction and should be reserved for patients with previous aortocoronary bypass surgery with use of the left internal mammary artery, critically stenosed, or hypoplastic right vertebral artery, or a functionally compromised circle of Willis, or in presence of an anatomical variant, such as an aberrant subclavian (lusorian) artery. We recommend careful preinterventional screening of the supra-aortic arteries with combined use of Doppler ultrasound and 3D MRA to confirm the presence of normally sized, nonhypoplastic vertebral arteries and an appropriate anatomical connection to the basilar artery.121
Extensive coverage of aortic segments has been reported to be a significant risk for spinal cord ischemia.122-126 Particularly, patients after repair of the abdominal aorta appear to be at risk because of interrupted spinal cord collateral blood supply secondary to ligation of lumbar arteries with previous open surgery.127-129 In those patients occlusion of the left subclavian artery without previous revascularization may contribute to an unpredictable risk of spinal cord ischemia as proximal collateral circulation via the anterior spinal artery, a branch of the ipsilateral vertebral artery, is jeopardized. In summary, observational evidence suggests that intentional LSA occlusion may be justified when required for proximal anchoring of stent-graft in the absence of supra-aortic vascular pathologies and previous aortic repair.130
HYBRID PROCEDURES FOR AORTIC ARCH PATHOLOGIES
The development of endovascular aortic surgery is characterized by the performance of increasingly complex procedures avoiding extended thoracotomy and extracorporeal circulation.131,132 The aortic arch morphology is challenging because of angulation and the proximity of the supra-aortic branches that need to be preserved. For traditional open arch reconstruction, using hypothermic cardiac arrest, extracorporeal circulation, and selective cerebral perfusion has been demonstrated to effectively assist in such major operations. However, open procedures for any arch pathology carry a high risk for in-hospital mortality (2%-9%) and neurological complications (4%-13%).133-135
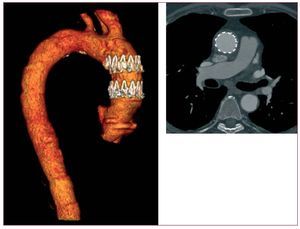
Therefore, classic surgery is often reserved for low-risk patients. As an alternative strategy, hybrid arch procedures (HAP) provide patient-centred solutions combining first stage debranching bypasses (to preserve cerebral perfusion) with a second stage endovascular exclusion of the affected arch. HAP is generally performed without hypothermic circulatory arrest or extracorporeal circulation and may expand the treatment group to older patients with severe comorbidities or redo-surgery currently ineligible for open surgical intervention. There are 2 different hybrid approaches with either extra-anatomic or intrathoracic supra-aortic vessel transposition. To treat distal arch aneurysms involving both the left subclavian and the left common carotid artery, those vessels can be translocated upstream to the right common carotid artery approached via cervical access (hemi-arch debranching).136,137 For arch aneurysms extending to the innominate artery the ascending aorta can be used, via sternotomy, as a donor site for debranching bypasses (total arch debranching) and will serve as proximal landing zone for the endograft.138,139 (Figure 11)
Figure 11. Aortic arch aneurysm in a 67 year-old man not eligible for classic surgical replacement. Hybrid approach included rerouting of the supraaortic branches with staged transfemoral implantation of an endovascular stent-graft across the aortic arch. Follow-up at 6 months revealed unimpaired bypasses and complete exclusion of the arch pathology (B).
ENDOLUMINAL "ELEPHANT TRUNK" COMPLETION IN EXTENSIVE AORTIC DISEASE
Thoracic aortic aneurysms affecting the arch and proximal descending aorta continue to challenge physicians. Today, single-stage operations have been widely replaced by a the 2-stage approach with initial placement of an "elephant trunk" under hypothermic circulary arrest followed by a second stage procedure for surgical extension of the graft to the distal aorta.140
Mortality rates of 4%-6% for the second-stage procedure were recently reported.141-143 Retrograde application of endovascular stent-grafts via femoral approach to complete the proximal procedure avoids the requisite thoracotomy and may improve the morbidity and mortality of the patient population at risk. In a recently published series of 22 patients Greenberg et al144 could demonstrate that mortality as well as neurological complications surrounding the endovascular procedure were exceptionally low, thus allowing safe performance of endovascular completion of elephant trunk repairs. Given the complex aortic morphology, it is beneficial to have access to tapered prostheses with active fixation systems, which allow accommodating disparate proximal and distal diameters, and similarly reducing the risk for caudal migration from pulsatile forces. The "elephant trunk" graft in general provides an adequate overlap for stent-graft insertion of at least 4 cm.144 However, excessive length and tortuosity of the "elephant trunk" graft can make the endovascular portion of the repair much more complicated and possibly less durable. Obviously, delaying the second-stage procedure will increase the risk of rupture, and thus, all efforts should be made to streamline the recovery from the first stage and complete the second stage expeditiously. Improvements in implant design and delivery systems will further simplify the second-stage portion of these complex aneurysm repairs. Endovascular elephant trunk completion may drastically diminish the complication rate in extended aortic repair, but is still in need of long-term results.
CONCLUSION AND FUTURE DIRECTIONS
The emerge of endovascular strategies as an alternative to open surgery constitutes an exciting field in patient care. Although it is apparent that high-risk patients will benefit from this technology, the exact role of stent-grafting remains to be defined as we continue to accumulate long-term data and as devices and techniques evolve. Instead of replacing conventional surgical treatment, endovascular repair will likely play a complementary role and offer a less invasive option in our treatment armamentarium. It is clear that limitations of both approaches are subject to change and the risk for open surgery somewhat subjective (judgement of comorbidities and physiological reserve), whereas contraindications for endovascular treatment are mainly defined by anatomical constraints. Current limitations of nonsurgical reconstruction will be addressed with new designs of highly individualized low-profile devices in order to expand applicability of stent-graft technology in the thoracic aorta. Interestingly, for both open and endovascular techniques in many scenarios the answer to the ethical question of justification of treatment is not in yet, because prospective data from randomized studies are not available nor are standard operational procedures or guidelines. However, if one insists on strict proof (or strict disproof) in empiric science, one will never benefit from experience and never learn from it how wrong one has been. Nevertheless, even in a world of rapidly advancing technology, it is ironically still wise to remember old principles of responsible use of clinical judgment and experience for the benefit of our patients. The growing segment of older patients with multiorgan comorbidities deserves a holistic approach, the intelligent use of prognosticating tools, and close interdisciplinary cooperation in a medicoethical framework.
Correspondence: C.A. Nienaber, MD, PhD, FACC, FESC,
Division of Cardiology, University Hospital Rostock, Rostock School of Medicine,
Ernst-Heydemann-Strasse 6, 18057 Rostock, Germany
E-mail: christoph.nienaber@med.uni-rostock.de