In 1987, Cox1 defined the technique and principles underlying one of the most effective treatments to date for atrial fibrillation (AF): the maze procedure. The procedure was designed to prevent atrial re-entries and enable sinus node impulses to activate the entire atrial myocardium in an orderly fashion. It involved creating a maze-like set of incisions for isolating or ablating clearly defined anatomic regions identified in previous animal studies.2
Iterations of the procedure seeking to reduce the risk of blockages led to the creation of the Cox-maze II and III procedures. The maze procedure was extremely effective, but it was technically complex and the instrumental limitations of having to cut and sew the atrial wall carried a risk of bleeding. The emergence of alternative energy sources made it possible to create similar lesion sets using radiofrequency and/or cryoablation (Cox-maze IV3), reducing the risks associated with the cut and sew technique and broadening the indications for the procedure.
The discovery by Haïsaguerre et al.4 in the 1990s that electric pulmonary vein isolation with radiofrequency catheter ablation was capable of eliminating AF led to an exponential growth of percutaneous approaches for the treatment of AF. The more aggressive surgical approach was reserved for patients already undergoing surgery for another coronary or structural defect.
In the past decade, however, there have been increasing reports of high atrial arrhythmia recurrence rates in patients with persistent and long-standing persistent AF following catheter ablation.5 Interest in stand-alone surgery for atrial arrhythmias has also been rekindled by the advent of new ablation energy sources and minimally invasive thoracoscopic procedures. The latest European guidelines on the diagnosis and management of AF recommend stand-alone thoracoscopic surgery for patients with paroxysmal or persistent AF refractory to antiarrhythmic drugs who experience recurrence or have a high risk of recurrence after catheter ablation (recommendation class/level of evidence IIa/B).6
Decisions on how to treat symptomatic patients with refractory AF or a high risk of recurrence despite optimal medical treatment and pulmonary vein isolation are complex.7 New technologies such as pulsed-field ablation8 have yielded very promising results, and the effects of targeted procedures such as ablation of AF drivers or mechanism-based strategies remain to be elucidated.9 Thoracoscopic AF surgery should not be viewed as an alternative to catheter ablation, in part because of conflicting evidence of its superiority in this setting.10 Rather, it should be seen as a complementary approach to be considered in the management of refractory or complex AF. It should also be included in the treatment algorithms of hospitals with extensive experience in this area.
In a recent article published in Revista Española de Cardiología, Wesselink et al.11 compared the risk of AF recurrence following minimally invasive thoracoscopic radiofrequency ablation between patients who had previously undergone catheter ablation and ablation-naïve patients. At 1-year of follow-up, the ablation-naïve patients were more likely to have achieved freedom from AF than those with a history of the procedure (72.5% vs 61.7%, P<.001). As argued in the article, previous catheter ablation is unlikely to induce sufficient atrial remodeling to influence the outcome of subsequent surgeries. It is much more likely that number of previous ablations is simply a risk marker or a variable on the pathway between AF duration/degree of atrial remodeling and unsuccessful surgical treatment. In short, patients with more complex and longer-standing AF would have been treated more often before being referred for surgery.
Hybrid interventions combining surgery and catheter ablation are becoming more common in patients with persistent or long-term persistent AF. Stand-alone pulmonary vein ablation offers limited results in this setting, but a hybrid approach can overcome the limitations of each technique used on its own and achieve more extensive and effective ablation of the entire posterior atrial wall, the ligament of Marshall, the appendage, the left isthmus, the superior vena cava, and the cavotricuspid isthmus, reproducing what would be a classic Cox maze lesion set.12
Stand-alone Thoracoscopic Ablation of AFThoracoscopic ablation is performed via a port approach (without sternotomy, thoracotomy, or extracorporeal circulation) in a traditional or hybrid operating room. The most widely used lesion set is designed to a) electrically isolate the posterior wall of the left atrium through the creation of a box lesion encompassing the mouth of the 4 pulmonary veins, b) resect the ligament of Marshall, and c) mechanically and electrically exclude the left atrial appendage through amputation with a clip or suture. A recent article described a new hybrid convergent procedure in which thoracoscopic ablation was limited to the left atrial posterior wall.13
Depending on factors such as AF duration, electrophysiological mapping, and previous procedures, other lesions can be created, such as ablation of the left isthmus via the anterior trigone (Dallas lesion set) or of the superior or inferior vena cava, the terminal crest, and the right atrial appendage.
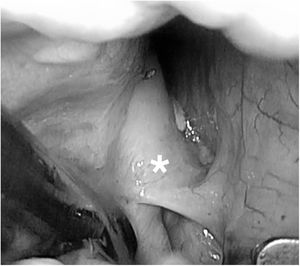
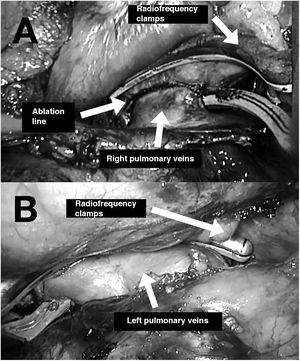
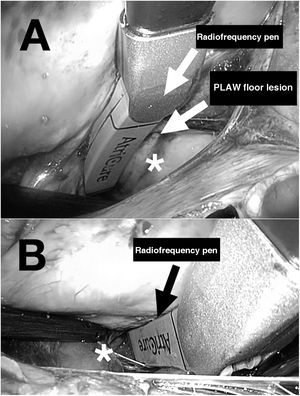
The two main types of ablation instruments used are radiofrequency clamps and pens. Their correct positioning requires surgical dissection of the pulmonary veins, the ligament of Marshall (figure 1), and the transverse and oblique pericardial sinuses. These procedures generally require prior surgical training due to the technical difficulty of the thoracoscopic approach. Ablation of the right and left pulmonary veins using bipolar radiofrequency clamps is illustrated in figure 2A,B. The creation of a lesion on the floor of the posterior left atrial wall connecting the inferior pulmonary veins through the transverse sinus is shown in figure 3A. Figure 3B shows the creation of a lesion between the superior pulmonary veins through the transverse sinus. Once the lesions are created, the entrance and exit block for the posterior left atrial wall and right and left pulmonary veins must be confirmed.
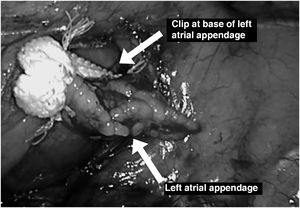
In all thoracoscopic AF ablation procedures, the atrial appendage must be excluded by clipping or automatic suture (figure 4), as mechanical occlusion has been shown not only to reduce the risk of thromboembolic events14 but also to provide electrical exclusion.15
Thoracoscopic ablation of AF is highly effective, with 5-year AF-free survival rates of more than 50% and an incidence of serious adverse events (eg, conversion to sternotomy, severe bleeding, stroke) of less than 1%.10
Impact of Previous Ablation on Thoracoscopic AF AblationIn their recent study in Revista Española de Cardiología, Wesselink et al.11 compared thoracoscopic AF ablation outcomes between ablation-naïve patients and patients with a previously failed ablation. The overall patient profile (66.6% with persistent or persistent long-standing AF, 41.5% aged > 65 years, AF duration of 4-6.5 years, markedly dilated atria, etc) justified the use of surgery rather than a catheter-based approach.
In the propensity score matching analysis, 72.5% of ablation-naïve patients were free of AF at 1 year compared with 61.1% of those with an prior unsuccessful catheter ablation (P=.034). It would be reasonable to assume that any ablation, regardless of the approach used (surgical or catheter-based), would be more likely to fail in patients with a history of failure. The question is whether the increased risk of failure is driven by a history of surgery or the presence of a more extensive, aggressive substrate. In other words, is prior ablation a risk factor per se, or rather a risk indicator or an intermediate variable linked to higher rates of AF treatment failure?
The authors themselves contemplated this possibility in their discussion, and based on an analysis of left atrial appendage collagen fiber densities, suggested that patients with previously failed ablations have more complex substrates. Obviously, the increased collagen densities observed are unlikely to have been caused by previous ablation, as catheter ablation of atrial appendages is rarely performed due to the risk of embolism.
In any event, a notable 46% of patients had undergone at least 2 catheter ablations before being referred for surgery, and 14.7% had undergone at least 3. One might wonder whether these patients should have been referred earlier for surgical ablation or evaluation at a referral hospital with expertise in complex AF.
Surgical treatment of AF is much less common in Spain than in other European countries or the United States. According to the Spanish Registry of Cardiovascular Surgery, just 23 lone thoracoscopic AF ablations were performed in 2019, and just 4% of patients undergoing surgery for another indication received surgical treatment of AF.16 We can only speculate as to why these figures are so low. Perhaps thoracoscopic ablation is reserved for very advanced stages of AF, or hospitals are unable to absorb the additional costs, or there may be a general lack of training among surgeons or a lack of familiarity with the procedure in the cardiology community.
Whatever the reason, it would appear that thoracoscopic AF ablation is not widely recommended or performed in Spain, despite evidence showing its added value when performed within integrated clinical, interventional, and surgical programs for patients with complex AF in centers of excellence.
FundingNone
Conflicts of InterestM. Carnero Alcázar is a proctor for AtriCure Europe, which manufactures surgical AF ablation devices and whose logo appears in some of the images in this article. L. Maroto-Castellanos and J.J. González-Ferrer declare that they have no conflicts of interest.
.