There is no doubt that sudden cardiac death (SCD) and ventricular arrhythmias (VA) are among the most devastating events affecting cardiovascular patients, families and even the general population. SCD worries most physicians dealing with risk stratification of patients, in a field full of uncertainties, but with very serious consequences in terms of actions to take and clinical outcomes. In the last few decades, the scientific community has recognized this field as a priority research area, speeding up the acquisition of new data, new technologies, new stratification models, and so on. Monthly, and even daily, new data are available for analysis. The consequence is that some randomized trials providing new evidence,1 but published almost simultaneously, were unfortunately not discussed in the new edition of the European Society of Cardiology (ESC) guidelines for the management of patients with VA and the prevention of SCD,2 which provides new recommendations that will definitely improve the care of patients at risk. The present editorial comment aims to highlight the most relevant innovations and novelties provided by the new guidelines.1
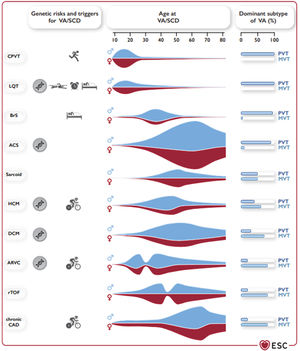
GENERAL ASPECTS AND CLINICAL EVALUATIONFollowing the patient-centered framework promoted by the ESC, the guidelines reinforce the importance of multidisciplinary teams and specialized centers for patient evaluation and interventional procedures (ie, catheter ablation, mechanical circulatory assist devices, and cardiothoracic surgical back-up). Contextualization is also well driven in the present guidelines. Given that half of SCDs occur as a first manifestation and that etiology can vary depending on many factors, there is a central figure (figure 1) which reflects genetic risk, the main triggers, age of clinical onset, and the dominant subtypes of VA. The document acknowledges the low survival rate after out-of-hospital cardiac arrest. For the first time, there are clear formal recommendations for community actions: a) the promotion of community training in basic life support (class I); b) the recommendation of prompt cardiopulmonary resuscitation by bystanders at out-of-hospital cardiac arrest (class I); c) the availability of appropriate access to automated external defibrillator at sites where cardiac arrest is more likely to occur (class I); and d) the existence of mobile phone-based alerting to assist nearby victims (class IIa).
Genetic risk for VA/SCD, typical triggers for VA/SCD, age at presentation with VA/SCD, sex predominance, and typical VA (PVT/VF vs MVT) in different diseases associated with VA/SCD. ACS, acute coronary syndrome; ARVC, arrhythmogenic right ventricular cardiomyopathy; BrS, Brugada syndrome; CAD, coronary artery disease; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LQT, long QT syndrome; MVT, monomorphic ventricular tachycardia; PVT, polymorphic ventricular tachycardia; rTOF, repaired tetralogy of Fallot; SCD, sudden cardiac death; VA, ventricular arrhythmia; VF, ventricular fibrillation. Reproduced with permission from Zeppenfeld et al.2.
For patient evaluation, advanced imaging techniques play a relevant role, including strain-rate imaging and cardiac magnetic resonance (CMR), with different techniques to study structural heart disease. Late gadolinium enhancement has been promoted as a relevant tool for diagnosis, risk stratification and even as a support for therapeutic procedures (ie, ventricular tachycardia [VT] ablation). Provocation tests have been also revisited with some relevant novelties. For example, a practical diagram displays recommendations for extended monitoring of patients undergoing pharmacological/provocation testing depending on the positivity of the result and the particular drug used for testing. In our media, most flecainide tests are performed in the out-patient clinic or during ambulatory hospitalization. However, the guidelines recommend 24-hour monitoring for positive flecainide testing, which might require a change to regular protocols in many hospitals. A possible alternative would be to make ajmaline available in Spain, which requires a much shorter monitoring period if positive. The latter would again require health authorities to make it possible, but clinicians are responsible for requesting it based on the new recommendations.
The authors have included a separate section for genetic testing. It is recommended to use next-generation sequencing with large panels. However, in routine diagnosis, these panels should ust include only candidate genes with a clear association with the studied disease and not questionable genes. Interpretation of the results is critical and there are no data supporting the benefit of mass screening programs in the general population. The task force also point out that genetic and clinical testing should be undertaken only by multidisciplinary teams with experience (class I). This aspect could represent a problem in scenarios other than multidisciplinary units. The increasing prevalence of these diseases hampers the referral of all these patients to tertiary centers, and therefore a network is required for feasible and realistic interaction between health care levels (local, regional, national, international).
Another novel approach is based on organization around 5 scenarios classifying the mode of clinical presentation. These scenarios are highly useful and illustrative, being organized into a) incidental finding of a nonsustained ventricular tachycardia (NSVT); b) first presentation of sustained monomorphic ventricular tachycardia (SMVT); c) sudden cardiac arrest survivor; d) SCD victim; and e) relatives of nonsurvivors of sudden arrhythmic death syndrome. In all of them, the importance of a family history is highlighted. In addition, CMR and genetic testing gain prominence. The role of urgent coronary angiography is extensively discussed, with the conclusion that, after several randomized control trials (1 provided by Spanish researchers3), there is no benefit in acute coronary syndrome without ST-segment elevation. However, a class I recommendation is supported in electrically unstable patients, with suspicion of ongoing myocardial ischemia. Coronary angiography may be complemented with a drug challenge to test vasospastic angina, but although this is an increasingly accepted technique in our environment, it was assigned only a class IIb recommendation. In the case of nonsurvivors of SCD, the important role of a comprehensive autopsy is emphasized, including blood/tissue collection for DNA extraction and genetic testing if the results of autopsy and toxicology are negative (class I). Translating that recommendation to our environment is a challenge that must be addressed, as only a minority of the Spanish territory adheres to guideline recommendations on molecular autopsy in nonsurvivors of SCD. This means that each autonomous community in Spain should facilitate a network of referral centers for the macroscopic, microscopic, and molecular study of the hearts and coordinate these centers with inherited cardiovascular disease units. At the same time, stable cooperative ties should be established between the justice departments (in charge of coroners and forensic pathologists), health administrations, and clinicians.
ACUTE MANAGEMENT OF VENTRICULAR ARRHYTHMIASAcute management is better described in the new guidelines. There is a new recommendation for prompt termination of SMVT, even if well-tolerated, as rapid hemodynamic deterioration may occur (class I).The PROCAMIO trial,4 performed in our environment by Ortiz M et al., has provided evidence for new strong recommendations to treat hemodynamically tolerated SMVT. If the etiology is unknown, intravenous procainamide is recommended ahead of amiodarone (class IIa vs IIb), with the exception of patients with severe heart failure, acute myocardial infarction, or end-stage renal disease. In addition, patients presenting with electrical storm are substantially addressed in the guidelines, including a very practical algorithm. Superficial or mid sedation is indicated as first-line therapy to alleviate psychological distress and decrease proarrhythmogenic sympathetic tone (class I). Regarding pharmacological treatment, nonselective beta-blockers, and specifically propranolol (more effective than metoprolol), gain significant attention combined with amiodarone as a first-line therapy. Landiolol, a new ultra-short-acting beta1-selective blocker, is proposed when VT is refractory to amiodarone, but it is not commercially available in Spain. Stepping forward, VT ablation is highly recommended (class I) for patients with electrical storm unresponsive to antiarrhythmic drugs (AAD), and probably superficial/mild sedation as well. Deep sedation is recommended but, interestingly, with a lower class (class IIa) than VT ablation. The documents then discusses how clinicians should proceed, indicating VT ablation prior to progression to deep sedation and mechanical ventilation, based on the stronger class of recommendation for VT ablation. The latter is supported by studies demonstrating improved rhythm control, survival and clinical outcomes with interventional electrophysiological procedures performed in experienced centers. It should also be considered in patients with recurrent episodes of polymorphic VT/ventricular fibrillation (VF) triggered by a similar premature ventricular complex (PVC), unresponsive to medical treatment or coronary revascularization (class IIa). Autonomic modulation is a novel alternative also gaining significant attention in clinical practice, but the evidence on its efficacy is inconclusive. Mechanical circulatory support may be considered in the management of drug-refractory electrical storm and cardiogenic shock, when conventional therapy fails, and to provide circulatory support during ablation (class IIb).
LONG-TERM MANAGEMENTIn the long-term, gross recommendations for secondary prevention remain mostly unchanged. However, some novelties that might have a significant clinical impact will be discussed later (see comments on specific structural heart diseases). Selection for an implantable cardioverter-defibrillator (ICD) in primary prevention is discussed elsewhere. This edition highlights the importance of adequate evaluation before implantation (and at the time of generator change), with special attention to life expectancy, quality of life and comorbidities, as they affect the expected benefits and risks. The document emphasizes that the final decision on implantation should be the result of a joint decision process. Assessment of psychological status and treatment, if needed, is recommended for all patients (class I). Discussion of issues related to device management at end-of-life is also given a class I indication. There are no new recommendations in patients with ischemic cardiomyopathy and severe systolic dysfunction compared with the previous guidelines. Unfortunately, ICD implantation in patients with nonischemic cardiomyopathy and severe systolic left ventricular dysfunction remains controversial. Overall, ICD implantation is recommended when left ventricular ejection fraction (LVEF) is less than 35% in patients with New York Heart Association class II (at least, avoiding class IV). However, the negative results of the DANISH trial continue fueling the debate, despite the proven survival benefit in patients younger than 70 years.5 A completely different picture is presented in the guidelines when dealing with dilated cardiomyopathy and hypokinetic nondilated cardiomyopathy secondary to inherited, inflammatory, infiltrative or neuromuscular etiology (see comments on specific structural heart diseases).
Programing of an ICD plays a relevant role in the guidelines, emphasizing the clinical benefits of longer detection times (8-12 s; 30 beats), higher rate cutoff values (188 vs 200 bpm) and longer windows for supraventricular rhythms discriminators (up to 230 bpm), which all together helps to reduce appropriate and inappropriate therapies with relevance for morbidity and mortality. One of the novel aspects of the guidelines concerns concomitant treatment (titration of beta-blockers) to avoid inappropriate therapies and/or invasive management in the prevention of inappropriate ICD therapy (catheter ablation is given a class I indication in patients with recurrent supraventricular tachycardia and atrial fibrillation resulting in inappropriate therapies). More recent technologies are also discussed. Regarding subcutaneous ICDs, the new guidelines include relevant data confirming noninferiority compared with transvenous ICDs (in patients with no need for bradycardia pacing, antitachycardia pacing, or resynchronization). However, the indications remain mostly unchanged. Overall, the same picture is observed with wearable cardiac defibrillators. The fact that these devices failed to improve survival in the early phase after myocardial infarction prevented any upgrading of indications. Although this is probably the most interesting scenario, their routine use cannot be recommended, but may be considered for selected patients (class IIb). However, the indication for patients in need of temporary protection is consolidated (ie, after removal of ICD because of infection, class IIa recommendation).
COMMENTS ON SPECIFIC STRUCTURAL HEART DISEASESAcute coronary syndromesUrgent reperfusion is the most important therapy for prevention of VA in ST-segment elevation myocardial infarction. Beta-blocker treatment is also recommended to prevent VA before revascularization (class I). Intravenous amiodarone should be considered to acutely suppress recurrent hemodynamically relevant VA (class IIa), although the evidence in this setting is mainly extrapolated from studies of out-hospital cardiac arrest. Lidocaine has a class IIb recommendation, to be considered only if treatment with beta-blockers and amiodarone is not effective after revascularization. The impact of VA occurring after reperfusion (> 48hours) on future SCD is less clear. The occurrence of VA late after reperfusion was associated with long-term all-cause mortality, while VA occurring early after reperfusion was not associated with 5-year outcomes. Further studies are required to clarify the impact of VA occurring>48hours after ST-elevation myocardial infarction on late SCD in contemporary patients undergoing acute PCI. These guidelines mention sudden cardiac arrest survivors with coronary artery spasm, in whom ICD implantation should be considered (class IIa) as medical intervention and multiple vasodilator drugs may not be sufficiently protective. Limited evidence suggests that invasive risk stratification by programmed electrical stimulation in the early postmyocardial infarction phase may be helpful for identification of high-risk patients with reduced LVEF (class IIb), but new evidence will be provided in the near future.
Chronic coronary syndromesThere are some important changes regarding patients with chronic coronary artery disease. First, the role of programmed electrical stimulation to test VT inducibility has been removed. In contrast, its use has been upgraded to class I in patients with a) previous myocardial infarction and unexplained syncope, and b) NSVT and LVEF between 36% to 40%. Second, the new guidelines have also updated some indications for ICD in primary prevention that were common practice but had not been reflected in the previous version. One of the most novel and relevant issues that directly impacts current practice refers to a particular subset of secondary prevention patients, those with hemodynamically tolerated SMVT who do not have severely impaired LVEF (≥ 40%). The lack of survival improvement shown by ICDs in this context and the potential SMVT abolition with catheter ablation opens up the possibility of withholding ICDs in such cases (class IIa) if catheter ablation is successful (ie, noninducible VT and elimination of abnormal electrograms). For the remaining patients with SMVT, an ICD is recommended (class I). One of the most expected issues for many readers is the way the guidelines deal with new evidence regarding the value provided by VT ablation procedures in the chronic phase of ischemic patients. While their use has been downgraded to class IIb as a preventive therapy after the first episode in persons without ICD, there are new recommendations for patients with recurrent SMVT despite chronic use of amiodarone (class I) or as first-line therapy (class IIa). Nevertheless, a recent trial published by Arenal et al.5 supports the use of catheter ablation before AAD for SMVT in patients with ischemic heart disease, providing better clinical results in the combined outcome of recurrent VT, hospital readmission, and death. This trial, conducted by Spanish researchers, was unfortunately not considered in the guidelines as it was published close to guideline publication. Probably, future recommendations should give stronger support of VT ablation for patients with ischemic heart disease.
Idiopathic premature ventricular complexesThe task force has considerably expanded the section on idiopathic PVCs and has provided numerous new recommendations. The guidelines emphasize the importance of excluding underlying structural heart disease in patients with PVCs and again encourage the use of CMR in atypical forms of presentation (ie, older age, right bundle branch block morphology, possible reentry) or inconclusive initial exams (new recommendation, class IIa, level C). Decisions are based on symptoms or deterioration of cardiac function. Treatment options differ according to the PVC origin. Catheter ablation is the preferred option for PVCs originating from the right ventricular outflow tract or the left ventricular fascicles, while beta-blockers (class I) and calcium channel blockers are the first choice for the remaining cases (class I). The guidelines mention the low risk of developing ventricular dysfunction in asymptomatic patients with a high burden of PVC and highlight the threshold of PVC>10% to cause cardiomyopathy, recommending regular follow-up only in these cases. Additionally, catheter ablation may be considered for selected patients with>20% of PVCs (class IIb). Due to its potential reversibility, the guidelines also underscore the importance of suspecting and recognizing PVC-induced cardiomyopathy in patients with PVC burden>10% and impaired cardiac function (class IIa) and again stress the role of CMR (class IIa). In this context, the role of catheter ablation has been upgraded to class I and is the preferred option over AAD. In patients with high PVC burden and aggravated structural heart disease (class IIa) or nonresponders to cardiac resynchronization therapy (class IIa), both catheter ablation and amiodarone are reasonable options.
CardiomyopathiesOf particular interest is the genetic basis of dilated and arrhythmogenic cardiomyopathies and its relationship with prognosis beyond LVEF. It is noticeable that in almost half of patients with apparently idiopathic dilated cardiomyopathy there is an underlying genetic etiology that can be easily identified. This is relevant given the different outcome depending on the genetic subtype.6 Apart from the already known poor arrhythmic prognosis of LMNA/C mutation carriers, some other gene targets have been added with an increased susceptibility to SCD in the presence of not severely reduced LVEF, such as FLNC. RBM20 and PLN, even though other high-arrhythmic risk genes (ie, desmosomal genes) are missing. Given the well-known overlap between dilated and arrhythmogenic cardiomyopathies, these new recommendations for a genetically tailored approach apply when a pathogenic mutation in these genes is detected, independently of the observed phenotype. In the specific case of LMNA/C mutation carriers, a risk calculator has been incorporated, considering some risk markers such as male sex, NSVT, and mildly impaired LVEF. In addition, some other risk factors have been added for the above -mentioned LMNA/C, RBM20 and PLN, including syncope, LGE and VT inducibility, in the presence of LVEF from 36% to 50%. On the other hand, with typical arrhythmogenic cardiomyopathy phenotypes, there is agreement that in cases with unexplained syncope or advanced structural heart disease with right, left, or biventricular involvement, an ICD should be considered. Overall, the spectrum of clinical variables influencing risk stratification has been expanded with differences between etiologies summarized in table 1.
Risk markers for sudden cardiac death in cardiomyopathies
| DCM | ACM | HCM | RCM | NCCM | Myotonic dystrophy | |
|---|---|---|---|---|---|---|
| LGE | +++ | ++ | ++ | + | + | + |
| Geneticsa | +++ | ++ | + | - | + | - |
| NSVT | ++ | ++ | +++ | - | - | + |
| Syncope | ++ | +++ | +++ | - | - | ++ |
| PES | + | + | + | - | - | ++b |
| AV block | ++ | - | - | - | - | ++ |
| Male sex | ++ | ++ | - | - | - | + |
–, no relationship;+, weak;++, strong;+++, very strong; ACM, arrhythmogenic cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; NCCM, noncompaction cardiomyopathy; PES, programmed electrical stimulation; RCM, restrictive cardiomyopathy.
In the case of hypertrophic cardiomyopathy (HCM), the classic risk stratification calculator is still valid in the new guidelines, with a special consideration in those cases in the “grey zone” with a 5-year risk of SCD between 4% and 6%. CMR can help to tilt the balance toward high risk in patients with a proportion of LGE>15%. Interestingly, for the first time the presence of a sarcomeric mutation is considered an additional factor to consider in these borderline cases. However, there is no mention to the different sarcomeric genes or specific mutations, so this advice seems somewhat incomplete. For minor forms of cardiomyopathy, such as noncompaction, restrictive or neuromuscular diseases, the lack of evidence allows only general recommendations from expert consensus.
Inflammatory, valvular, and congenital cardiac diseasesVT ablation is increasingly recognized as a therapeutic tool in chronic myocarditis with recurrent SMVT or ICD shocks with little response to AAD (class IIa) and, for the first time, it is considered an alternative to ICD for well-tolerated VT with preserved LVEF function and limited scar (class IIb). A new recommendation has been provided for ICD implantation in patients with hemodynamically not tolerated VT/VF during the acute phase of myocarditis before hospital discharge (class IIa), in addition to previous recommendation in the chronic phase. A new algorithm for SCD prevention and treatment of VA in cardiac sarcoidosis is introduced in the present guidelines, based primarily on CMR and electrophysiology study findings. An ICD is recommended in any of the following scenarios: aborted cardiac arrest or SVMT (class I), LVEF<35% (class I), significant LGE at CMR after resolution of acute inflammation (class IIa), indication for permanent pacing related to high-degree AV block (class IIa). In the case of LVEF 35% to 50% and minor LGE, SMVT inducibility at programmed electrical stimulation is an additional criterion for ICD implantation (class IIa). The definition of significant LGE remains elusive. Although VT ablation may be useful for patients with VA, it is only recommended after AAD failure (class IIb). In Chagas cardiomyopathy, amiodarone and catheter ablation have demonstrated efficacy to control recurrent VA in symptomatic patients. A new recommendation is provided for both (class IIa, AAD are considered first choice). Surprisingly, the benefit of ICD in improving prognosis remains controversial, even for secondary prevention. Recommendations for ICD have therefore been downgraded: ICD is only recommended in patients with symptomatic VT if AAD are ineffective (class IIb).
In valvular disease, increasing attention is paid to the association between SCD and mitral valve prolapse and the potential role of CMR in risk stratification. The need for studies focused on identification of the subgroup of patients at risk of SCD is highlighted.
The document emphasizes assessment of residual lesions or new structural abnormalities in patients with congenital heart disease and sustained VA (class I). After recent publications linking supraventricular tachycardia with fast ventricular conduction to SCD (especially in patients with atrial switch for transposition of the great arteries, Fontan operation and Ebstein anomaly), a new recommendation is provided for treatment with cardiac ablation in selected patients with cardiac arrest (class IIa). Separate recommendations are provided for patients with repaired tetralogy of Fallot. Although programmed electrical stimulation remains strongly recommended for arrhythmic risk stratification in symptomatic patients and NSVT (class IIa), its recommendation in asymptomatic patients has been downgraded (class IIb). Finally, preoperative transection of VT-related anatomical isthmuses in patients with SMVT undergoing surgical or percutaneous pulmonary vein replacement is encouraged (class IIa). However, the potential usefulness of this approach in asymptomatic patients is not discussed.
RECOMMENDATION ON PRIMARY ELECTRICAL DISEASEUnfortunately, due to the lack of strong evidence on inherited primary arrhythmia syndromes, most recommendations continue to have level of evidence C. A remarkable positive aspect in the current guidelines is the inclusion of clinical management algorithms that integrate the most relevant recommendations for the various clinical scenarios. Genetic testing is highlighted in the new guidelines, having a class I indication in most channelopathies.
Although idiopathic VF remains a diagnosis of exclusion, it has been included in the current guidelines among primary electrical diseases. Genetic testing and clinical evaluation of first-degree family members can be considered (class IIb). Isoproterenol, quinidine or verapamil for acute treatment of electrical storm and quinidine for chronic therapy to treat recurrent ICD shocks are recommended (class IIa). The level of recommendation for catheter ablation in experienced centers of PVC inducing recurrent VF has dropped from I to IIa in the current guidelines. As in previous guidelines, cutoff points of QT ≥ 480 and ≥ 460 msec are recommended for the diagnosis of long QT syndrome (LQTS) in asymptomatic and symptomatic patients, respectively. Routine use of the epinephrine test is now discouraged (class III), due to its poor reproducibility. However, the usefulness of the “standing test” in the diagnosis of LQTS is emphasized. Beta-blockers continue to be recommended in all patients with LQTS. The new version of the guidelines indicates the preference for nonselective beta-blockers such as nadolol and propranolol. The recommendation for mexiletine in LQTS type 3 has been upgraded from class IIb to class I. Unfortunately, there are limitations in Spain to following these recommendations due to limited access to these drugs: propranolol retard (which facilitates therapeutic adherence) is no longer available and nadolol and mexiletine require request as foreign drugs. Andersen-Tawil syndrome is presented for the first time in the guidelines in a separate section. This change is positive given the specific characteristics of the disease. Beta-blockers and/or flecainide are the drugs of choice to treat VA. ICD is recommended after cardiac arrest or not tolerated VT, and implantable loop recording should be considered if there is unexplained syncope. With regards to short QT syndrome, a very important change has been made in the diagnostic criteria, such as<360 msec and a pathogenic mutation or clinical features (family history or survival from a VT/VF episode) are the only class I criteria. Additionally, a cutoff of 320 msec (not 340 msec) can be used as single diagnostic criterion (class IIa). Finally, genetic testing is emphasized with level of recommendation I. It must be considered, however, that all recommendations are based on expert consensus and therefore more evidence is needed in the future.
Possibly the most controversial aspect in Brugada syndrome (BrS) is the modification of diagnostic criteria. In patients with an induced Brugada pattern, the presence of other clinical features, such as documented polymorphic VT/VF, arrhythmic syncope, or a family history of BrS or SCD (< 45 years), is required for diagnosis. This change has been justified by the lower specificity of provocation tests than initially thought. General and follow-up recommendations on asymptomatic patients with a positive pharmacological test are lacking. The indication for ICD in secondary prevention and in patients with arrhythmic syncope remains unchanged. Risk stratification in asymptomatic patients is challenging and the subject of a long debate. The current guidelines have limited the consideration of electrophysiological study (EPS) for risk stratification to asymptomatic patients with spontaneous type 1 Brugada pattern using a protocol including up to 2 extrastimuli. The recommendation for catheter ablation (epicardial substrate or triggering PVCs) in patients with recurrent ICD shocks refractory to quinidine has been upgraded from class IIb to IIa. For the first time, recommendations are made in early repolarization syndrome, a closely related entity to Brugada syndrome. Isoproterenol and quinidine are recommended for the treatment of patients with arrhythmic storm and recurrent ICD shocks, respectively. An implantable loop recorder should be considered (class IIa) in patients with early repolarization pattern and arrhythmic syncope. More controversial is the recommendation (class IIb) of ICD or quinidine for patients with early repolarization pattern, arrhythmic syncope, and additional risk features such as a high-risk electrocardiogram pattern or a family history of early repolarization syndrome or juvenile unexplained SCD.
Regarding catecholaminergic polymorphic ventricular tachycardia, 2 main features should be discussed. First, a very illustrative flowchart for the management of these patients has been included with different scenarios and the level of recommendation in each situation. Second, some recommendations have changed. In this regard, the role of genetic testing has been updated (class I), beta-blockers are now recommended also in all genetically positive patients despite no phenotype expression (class IIa) and the superiority of nonselective beta-blockers (nadolol or propranolol) has been emphasized. Finally, the role of left cardiac sympathetic denervation has been reviewed and, although the document states that it cannot substitute ICD implantation, the level of recommendation based on recent large publications has been updated to IIa in cases of beta-blockers and flecainide failure.
RECOMMENDATION ON OTHER CONDITIONSRecommendations on the management of arrhythmias during pregnancy have not changed substantially but catheter ablation (using nonfluoroscopic mapping systems) has been included as a class IIa recommendation. Moreover, the potential role of wearable cardioverter-defibrillators is discussed in patients with peripartum cardiomyopathy while awaiting LVEF recovery. In this regard, a new indication has also been included (class IIb) in patients awaiting heart transplant. The potential risk of VA is also reviewed in 2 other specific groups: athletes and older adults. Current guidelines have opted for a more practical approach, with a clear differentiation according to young (< 35 years) or middle-aged athletes (> 35 years old). The recommendations of cardiopulmonary resuscitation training and automated external defibrillation in sport facilities have been upgraded from class IIa to class I. Finally, the frequent controversy about age and the risk of SCD remains unsolved. The guidelines state that collective data from randomized and observational studies indicate that the benefit of ICDs is unclear in patients>75 years. However, a clear recommendation on age is not given and personalized assessment is recommended.
CONCLUSIONSAlthough significant gaps in evidence remain, the present guidelines provide new recommendations for risk stratification and treatment. Several advances have been recognized, especially in inherited, inflammatory, and infiltrative disorders. However, new and promising recommendations are provided for more classic clinical scenarios, which will hopefully result in better patient care.
FUNDINGNone.
CONFLICTS OF INTERESTThe conflict-of-interest declaration documents of all authors can be seen in the supplementary data.
SEC Working Group for the 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Rut Andrea Riba (coordinator), David Calvo Cuervo (coordinator), Almudena Aguilera Saborido, Pablo Ávila Alonso, Roberto Barriales Villa, Susana Bombín González, Victoria Cañadas Godoy, Juan Fernández Armenta, Juan Jiménez Jaimez, Esther Pérez David, Ivo Roca Luque, Ana Viana Tejedor.
SEC Guidelines Committee: Rut Andrea, Pablo Avanzas, Gemma Berga, Araceli Boraita, David Calvo, Raquel Campuzano, Victoria Delgado, Laura Dos Subirá, Juan José Gómez Doblas, Pilar Mazón, Domingo Pascual, Juan Sanchis, José M. de la Torre, David Vivas, José L. Ferreiro (president).
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.11.008
The names of all the authors of the article are listed in alphabetical order in Appendix A.
See related article: https://secardiologia.es/cientifico/guias-clinicas/arritmias/13800-2022-esc-guidelines-for-the-management-of-patients-with-ventricular-arrhythmias-and-the-prevention-of-sudden-cardiac-death.
Corresponding author.
Email addresses: davidcalvo307@gmail.com (D. Calvo); randrea@clinic.cat (R. Andrea).