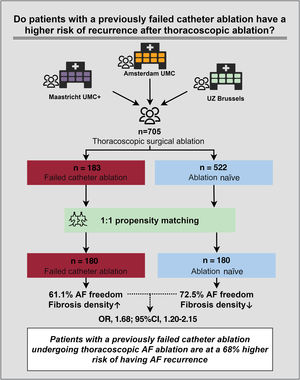
Recent observations suggest that patients with a previous failed catheter ablation have an increased risk of atrial fibrillation (AF) recurrence after subsequent thoracoscopic AF ablation. We assessed the risk of AF recurrence in patients with a previous failed catheter ablation undergoing thoracoscopic ablation.
MethodsWe included patients from 3 medical centers. To correct for potential heterogeneity, we performed propensity matching to compare AF freedom (freedom from any atrial tachyarrhythmia> 30 s during 1-year follow-up). Left atrial appendage tissue was analyzed for collagen distribution.
ResultsA total of 705 patients were included, and 183 had a previous failed catheter ablation. These patients had fewer risk factors for AF recurrence than ablation naïve controls: smaller indexed left atrial volume (40.9± 12.5 vs 43.0±12.5 mL/m2, P=.048), less congestive heart failure (1.5% vs 8.9%, P=.001), and less persistent AF (52.2% vs 60.3%, P=.067). However, AF history duration was longer in patients with a previous failed catheter ablation (6.5 [4-10.5] vs 4 [2-8] years; P<.001). In propensity matched analysis, patients with a failed catheter ablation were at a 68% higher AF recurrence risk (OR, 1.68; 95%CI, 1.20-2.15; P=.034). AF freedom was 61.1% in patients with a previous failed catheter ablation vs 72.5% in ablation naïve matched controls. On histology of the left atrial appendage (n=198), patients with a failed catheter ablation had a higher density of collagen fibers.
ConclusionsPatients with a prior failed catheter ablation had fewer risk factors for AF recurrence but more frequently had AF recurrence after thoracoscopic AF ablation than ablation naïve patients. This may in part be explained by more progressed, subclinical, atrial fibrosis formation.
Keywords
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with an estimated incidence of 2% to 4% in adults.1 AF is associated with a 2- to 3-fold increased mortality, an increased risk of stroke, heart failure and dementia, and an overall decreased quality of life.1,2 In patients with symptomatic AF, rhythm control therapy with antiarrhythmic drugs (AAD) or ablation is indicated.2 Catheter ablation is the most commonly used ablation therapy for AF.2 Thoracoscopic surgical ablation is a safe and effective alternative, particularly for patients with advanced AF.3–5 Thoracoscopic ablation may be indicated after failure of initial catheter ablation. However, previous research has shown that patients with a previous failed catheter ablation had a higher risk of recurrent AF after thoracoscopic ablation after 5 years of follow-up.6 Conversely, Lim et al.7 showed a similar rate of AF recurrence between patients with and without a previous catheter ablation. However, patients with a previous failed catheter ablation were younger, had smaller left atrial volume index (LAVI) and fewer patients had persistent AF compared with those without a previous catheter ablation. These baseline differences may have concealed expected differences in outcomes. Taking these studies into consideration, patients with a previous failed catheter ablation seem to demonstrate an increased risk of recurrent AF after thoracoscopic ablation.
Therefore, we hypothesized that patients with a previously failed catheter ablation are at higher risk of recurrent AF. This hypothesis cannot be tested with a randomized design. We therefore studied the outcome of patients undergoing thoracoscopic ablation for symptomatic AF with a propensity score matched analysis (with replacement of controls).
METHODSPatient selectionWe included patients undergoing their first thoracoscopic ablation from 3 University Medical Centers between May 2008 and May 2019. Based on a small pilot study, we expected a 10% difference in AF recurrence between matched patients with and without a previous failed catheter ablation. Using the McNemar paired test, alpha=0.05 and 80% power, we calculated that we needed at least 148 matched pairs to demonstrate a significant difference. Baseline clinical characteristics prior to the thoracoscopic treatment, including history of ablations and left atrial size, were collected. Patients referred for thoracoscopic or hybrid ablation for advanced AF have failed therapy with at least 1 antiarrhythmic drug. Patients usually have persistent AF, an enlarged left atrium, prior failed catheter ablation, or patient preference instead of a catheter ablation.4,8,9 The study was conducted according to the criteria set by the declaration of Helsinki.10 The ethics committee of the Amsterdam UMC reviewed the study protocol, gave permission to perform this study, and confirmed that the “Medical Research Involving Human Subjects Act” does not apply to this study (W22_077 # 22.110app.). A waiver for obtaining informed consent was provided since this was a retrospective trial, patients were not subjected to an intervention, and data were anonymously collected. Data cannot be shared for ethical and privacy reasons. Data may be shared upon reasonable request to the corresponding author.
From prospectively collected databases of the Amsterdam UMC, Maastricht UMC+and UZ Brussels, patients with a previous failed catheter ablation and ablation naïve controls were identified and included in the current analysis. All patients underwent epicardial ablation with epicardial or endocardial evaluation of conduction block. In the Amsterdam UMC, all patients underwent totally thoracoscopic ablation with pulmonary vein isolation, and additional roofline and trigone line in patients with persistent AF. In the Maastricht UMC+ and UZ Brussels, patients underwent simultaneous (same day) epi- and endocardial ablation with a stepwise approach. If AF was present or inducible after pulmonary vein isolation (PVI), a box lesion was applied epicardially. A cavotricuspid isthmus line and/or mitral isthmus lesion was applied if there was typical flutter and an intercaval lesion was applied in patients with a dilated right atrium. During endocardial evaluation of conduction block, additional endocardial touch up was performed when conduction block was not achieved epicardially as described previously.8,9,11
Follow-up and endpointsFollow-up was performed with 24-hour Holter monitoring at 3, 6, 9 and 12 months. Additionally, registration of symptomatic episodes and cardioversions were collected. The primary endpoint of the study was freedom of any atrial tachyarrhythmia> 30 s without the use of AADs during 1-year follow-up, according to the 2017 HRS/EHRA/ECAS/APHRS/SOLEACE consensus statement.2 During the blanking period of 90 days after the procedure, recurrences were not considered a failure of the procedure. AADs were discontinued 3 months after the procedure. The primary endpoint was freedom from AF without the use of AADs after 1 year, with exclusion of the first 3 months, which were considered the blanking period.12
Histological analysisLeft atrial appendage (LAA) histology was available from a selection of patients from the Amsterdam UMC. These patients all provided written informed consent for the use of LAA tissue for histologic analysis. The LAA was fixed in 4% formalin and embedded in paraffin. Sections of 5 μm thickness were prepared and stained with Picrosirius red for interstitial collagen quantification. Sections were digitized at 40 x magnification (Philips IntelliSite Ultra Fast Scanner, 0.25 μm/pixel, Philips, The Netherlands) and 20 nonoverlapping fields (maximal 5000 by 5000 pixels) from each patient were randomly selected for interstitial collagen quantification. Endocardial, epicardial and perivascular collagen were manually excluded. An automated image analysis using Image J Software color deconvolution was used to determine the area fraction of collagen of the combined area of cardiomyocytes and collagens with exclusion of white background in the images.
All individual interstitial collagen fibers were quantified using a custom Matlab-based algorithm. Images were binarized to a black-and-white image after color deconvolution, preprocessed and filtered. First, all white pixels (representing collagen) were dilated and eroded using a 5 pixel (1.25 μm) radius stamp to smooth the image. Holes of maximal 2000 pixels were filled. Large collagen fibrils (> 1250 μm2) were identified, quantified and removed from the image. The length of each remaining fiber was determined by morphological skeletonization. This technique creates a 2-dimensional skeleton. The fiber length of each skeleton ≥ 4 pixels was defined as the length between the 2 end points that were farthest apart. The fiber area was determined by the number of pixels. We quantified the number of individual fibers, and the fiber density, defined as the total number of fibers divided by the total area of myocardium and interstitial collagen combined. All histological analyses were performed blinded to clinical status and outcome. An illustrative example of the fiber analysis is shown in figure 1 of the supplementary data.13
Statistical analysisWe performed 2 propensity-based analyses. The main analysis was propensity score matching with replacement of controls. We performed nearest neighbor matching based on the propensity score; the caliper was 0.1 standard deviation of the propensity score. Matching was performed with replacement, implying that 1 ablation naïve control could be matched to more than 1 case with previous failed ablation. The differential risk of AF recurrence of the matched participants was assessed with the paired McNemar test. Matching was repeated 20 times and the results were pooled using Rubin's rules. Double adjustment of the primary analysis by logistic regression with clustered standard errors was performed, including all variables with standardized mean difference> 0.1 after matching. Conditional logistic regression was used as a secondary analysis to further assess the association between a previous failed catheter ablation and freedom from AF. This analysis corrects for confounders based on the propensity score and allows the inclusion of all patients in whom a propensity score was calculated. Conditional logistic regression was performed with time=1, 20 strata, and robust variance calculation.
Propensity scores were calculated with a logistic regression model with previous catheter ablation as the dependent outcome and 17 independent variables. A predefined set of variables was used, with addition of variables that were significantly associated with freedom from AF (P <.05 in univariable Cox regression analysis). The selected variables were sex, age, diabetes mellitus, stroke, hypertension, vascular disease, AF type, LAVI, AF history duration (log transformed), body mass index (BMI), history of congestive heart disease, mitral valve insufficiency, left atrial appendage (LAA) exclusion, lesion set of thoracoscopic ablation (categorized as ‘PVI only’, ‘PVI+Dallas lesion set’, ‘PVI+box lesion’), ganglion plexus ablation during the procedure, and the center where thoracoscopic ablation was performed. The definitions and details of included variables are shown in table 1 of the supplementary data. The rhythm outcome was defined according to current guidelines: freedom from any atrial tachyarrhythmia> 30s on electrocardiogram or Holter recording, without the use of AADs2.
Baseline characteristics of the total cohort were compared using the Student t test and Mann-Whitney U test for normally and nonnormally distributed continuous variables, respectively. The chi-square test was used to compare binary and categorical variables. The distribution of collagen fiber thickness was compared with the Kolmogorov-Smirnov 2 sample test. All tests were 2-sided and a P-value <.05 was considered to represent statistical significance.
Patient and public involvementNeither patients nor the public were involved in the design, conduct, or reporting of this study.
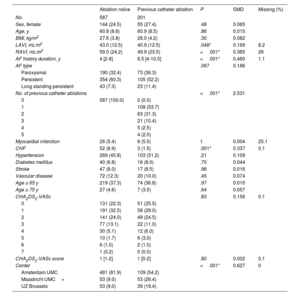
RESULTSPatient cohort and baseline characteristicsA total of 788 patients undergoing thoracoscopic surgical ablation for AF were available for this analysis: 590 patients from Amsterdam UMC, 106 from Maastricht UMC+, and 92 from UZ Brussels. The baseline characteristics of all patients are shown in table 1. Patients with a previous failed catheter ablation had undergone 1 (n=108; 54%), 2 (n=63; 31%), 3 (n=21; 10%), 4 (n=5; 3%) or 5 (n=4; 2%) earlier ablations. We included 109, 53 and 39 patients with a failed catheter ablation from Amsterdam UMC, Maastricht UMC+and UZ Brussels, respectively.
Baseline characteristics
| Ablation naïve | Previous catheter ablation | P | SMD | Missing (%) | |
|---|---|---|---|---|---|
| No. | 587 | 201 | |||
| Sex, female | 144 (24.5) | 55 (27.4) | .48 | 0.065 | |
| Age, y | 60.8 (8.8) | 60.9 (8.5) | .86 | 0.015 | |
| BMI, kg/m2 | 27.6 (3.8) | 28.0 (4.2) | .30 | 0.082 | |
| LAVI, mL/m2 | 43.0 (12.5) | 40.9 (12.5) | .048* | 0.169 | 8.2 |
| RAVI, mL/m2 | 59.0 (24.2) | 49.9 (23.5) | <.001* | 0.385 | 26 |
| AF history duration, y | 4 [2-8] | 6.5 [4-10.5] | <.001* | 0.460 | 1.1 |
| AF type | .067 | 0.186 | |||
| Paroxysmal | 190 (32.4) | 73 (36.3) | |||
| Persistent | 354 (60.3) | 105 (52.2) | |||
| Long standing persistent | 43 (7.3) | 23 (11.4) | |||
| No. of previous catheter ablations | <.001* | 2.531 | |||
| 0 | 587 (100.0) | 0 (0.0) | |||
| 1 | 108 (53.7) | ||||
| 2 | 63 (31.3) | ||||
| 3 | 21 (10.4) | ||||
| 4 | 5 (2.5) | ||||
| 5 | 4 (2.0) | ||||
| Myocardial infarction | 26 (5.4) | 6 (5.5) | 1 | 0.004 | 25.1 |
| CHF | 52 (8.9) | 3 (1.5) | .001* | 0.337 | 0.1 |
| Hypertension | 269 (45.8) | 103 (51.2) | .21 | 0.109 | |
| Diabetes mellitus | 40 (6.8) | 16 (8.0) | .70 | 0.044 | |
| Stroke | 47 (8.0) | 17 (8.5) | .96 | 0.016 | |
| Vascular disease | 72 (12.3) | 20 (10.0) | .45 | 0.074 | |
| Age ≥ 65 y | 219 (37.3) | 74 (36.8) | .97 | 0.010 | |
| Age ≥ 75 y | 27 (4.6) | 7 (3.5) | .64 | 0.057 | |
| CHA2DS2-VASc | .83 | 0.156 | 0.1 | ||
| 0 | 131 (22.3) | 51 (25.5) | |||
| 1 | 191 (32.5) | 58 (29.0) | |||
| 2 | 141 (24.0) | 49 (24.5) | |||
| 3 | 77 (13.1) | 22 (11.0) | |||
| 4 | 30 (5.1) | 12 (6.0) | |||
| 5 | 10 (1.7) | 6 (3.0) | |||
| 6 | 6 (1.0) | 2 (1.0) | |||
| 7 | 1 (0.2) | 0 (0.0) | |||
| CHA2DS2-VASc score | 1 [1-2] | 1 [0-2] | .80 | 0.002 | 0.1 |
| Center | <.001* | 0.627 | 0 | ||
| Amsterdam UMC | 481 (81.9) | 109 (54.2) | |||
| Maastricht UMC+ | 53 (9.0) | 53 (26.4) | |||
| UZ Brussels | 53 (9.0) | 39 (19.4) |
AF, atrial fibrillation; BMI, body mass index; CHF, congestive heart failure; LAVI, left atrial volume index; RAVI, right atrial volume index; SMD, standardized mean difference.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
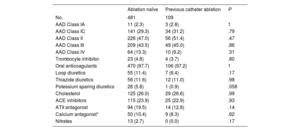
The baseline characteristics are shown in table 1. Patients with a previous failed catheter ablation had a longer history of AF compared with ablation naïve patients: 6.5 [4-10.5] vs 4 [2-8] (P=<.001) years. However, patients with a failed catheter ablation had a smaller LAVI (40.9±12.5 vs 43.0±12.5mL/m2, P=.048), less congestive heart failure (1.5% vs 8.9%, P=.001), and a numerically lower proportion had persistent AF (52.2% vs 60.3%, P=.067) at the time of the thoracoscopic ablation. The data from the Amsterdam UMC showed that there were no differences in the use of antiarrhythmic or other relevant cardiovascular drugs (table 2).
Baseline antiarrhythmic and other cardiac drug use of the patients from the Amsterdam UMC
| Ablation naïve | Previous catheter ablation | P | |
|---|---|---|---|
| No. | 481 | 109 | |
| AAD Class IA | 11 (2.3) | 3 (2.8) | 1 |
| AAD Class IC | 141 (29.3) | 34 (31.2) | .79 |
| AAD Class II | 226 (47.0) | 56 (51.4) | .47 |
| AAD Class III | 209 (43.5) | 49 (45.0) | .86 |
| AAD Class IV | 64 (13.3) | 10 (9.2) | .31 |
| Trombocyte inhibitor | 23 (4.8) | 4 (3.7) | .80 |
| Oral anticoagulants | 470 (97.7) | 106 (97.2) | 1 |
| Loop diuretics | 55 (11.4) | 7 (6.4) | .17 |
| Thiazide diuretics | 56 (11.6) | 12 (11.0) | .98 |
| Potessium sparing diuretics | 28 (5.8) | 1 (0.9) | .058 |
| Cholesterol | 125 (26.0) | 29 (26.6) | .99 |
| ACE inhibitors | 115 (23.9) | 25 (22.9) | .93 |
| ATII antagonist | 94 (19.5) | 14 (12.8) | .14 |
| Calcium antagonist* | 50 (10.4) | 9 (8.3) | .62 |
| Nitrates | 13 (2.7) | 0 (0.0) | .17 |
AAD, antiarrhythmic drug; ACE, angiotensin-converting-enzyme.
Data are expressed as No. (%).
All patients underwent thoracoscopic ablation with bilateral PVI. Additional roof- and trigone lesion were applied in 307 (39.0%) patients and a posterior box was performed in 186 (23.6%) patients. A total of 184 (23.4) patients underwent catheterization as part of the hybrid procedure. In 34 (18.5%) participants, touch up ablation was performed on one of the epicardially applied lesions. In 37 (20.1%), a cavotricuspid isthmus line was made, in 27 (14.7%) a mitral isthmus line, and in 68 (37.0%) patients complex fractionated atrial electrogram-ablation.
For calculation of the propensity score, missing data of one of the covariables resulted in removal of 83 participants (missing outcome n=10; LAVI n=63; AF history duration n=8; myocardial infarction n=1; CHA2DS2-VASc score n=1). In total, 705 patients were included in the primary analysis; 183 cases with a failed catheter ablation and 522 ablation naïve controls (figure 1).
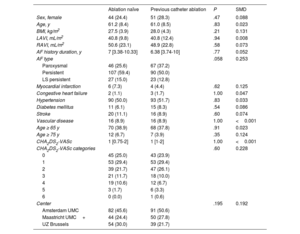
Primary outcomeIn the propensity matched analysis, with replacement of controls, 180 cases with a previous failed catheter ablation were matched to 180 ablation naïve controls (table 3). Freedom from AF was 61.1% in patients with a previous catheter ablation compared with 72.5% in ablation naïve matched controls. Patients with a previous catheter ablation were at a 68% increased risk of AF recurrence (OR = 1.68; 95%CI, 1.20-2.15; P=.034) (table 2 of supplementary data). Distribution of the propensity scores before and after matching are shown in figure 2 of the supplementary data. In the secondary analysis, we included all 705 patients in whom a propensity score was calculated. Conditional logistic regression analysis demonstrated 39% increased risk of AF recurrence (OR = 1.39; 95%CI, 1.01–1.92; P=.046) (table 4).
Baseline characteristics after propensity score matching 180 cases with replacement of controls
| Ablation naïve | Previous catheter ablation | P | SMD | |
|---|---|---|---|---|
| Sex, female | 44 (24.4) | 51 (28.3) | .47 | 0.088 |
| Age, y | 61.2 (8.4) | 61.0 (8.5) | .83 | 0.023 |
| BMI, kg/m2 | 27.5 (3.9) | 28.0 (4.3) | .21 | 0.131 |
| LAVI, mL/m2 | 40.8 (9.8) | 40.8 (12.4) | .94 | 0.008 |
| RAVI, mL/m2 | 50.6 (23.1) | 48.9 (22.8) | .58 | 0.073 |
| AF history duration, y | 7 [3.38-10.33] | 6.38 [3.74-10] | .77 | 0.052 |
| AF type | .058 | 0.253 | ||
| Paroxysmal | 46 (25.6) | 67 (37.2) | ||
| Persistent | 107 (59.4) | 90 (50.0) | ||
| LS persistent | 27 (15.0) | 23 (12.8) | ||
| Myocardial infarction | 6 (7.3) | 4 (4.4) | .62 | 0.125 |
| Congestive heart failure | 2 (1.1) | 3 (1.7) | 1.00 | 0.047 |
| Hypertension | 90 (50.0) | 93 (51.7) | .83 | 0.033 |
| Diabetes mellitus | 11 (6.1) | 15 (8.3) | .54 | 0.086 |
| Stroke | 20 (11.1) | 16 (8.9) | .60 | 0.074 |
| Vascular disease | 16 (8.9) | 16 (8.9) | 1.00 | <0.001 |
| Age ≥ 65 y | 70 (38.9) | 68 (37.8) | .91 | 0.023 |
| Age ≥ 75 y | 12 (6.7) | 7 (3.9) | .35 | 0.124 |
| CHA2DS2-VASc | 1 [0.75-2] | 1 [1-2] | 1.00 | <0.001 |
| CHA2DS2-VASc categories | .60 | 0.228 | ||
| 0 | 45 (25.0) | 43 (23.9) | ||
| 1 | 53 (29.4) | 53 (29.4) | ||
| 2 | 39 (21.7) | 47 (26.1) | ||
| 3 | 21 (11.7) | 18 (10.0) | ||
| 4 | 19 (10.6) | 12 (6.7) | ||
| 5 | 3 (1.7) | 6 (3.3) | ||
| 6 | 0 (0.0) | 1 (0.6) | ||
| Center | .195 | 0.192 | ||
| Amsterdam UMC | 82 (45.6) | 91 (50.6) | ||
| Maastricht UMC+ | 44 (24.4) | 50 (27.8) | ||
| UZ Brussels | 54 (30.0) | 39 (21.7) |
AF, atrial fibrillation; BMI, body mass index; CHF, congestive heart failure; LS, long standing; LAVI, left atrial volume index; RAVI, right atrial volume index; SMD, standardized mean difference.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Double adjustment of the primary analysis for suboptimally balanced variables (AF type, BMI, age ≥ 75, CHA2DS2-VASc score and treatment center) resulted in OR = 1.49; 95%CI, 1.03-2.15; P=.035 for patients with a previous failed catheter ablation compared with ablation naïve controls.
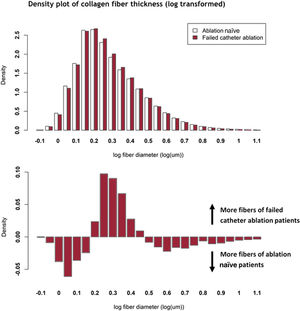
Atrial fibrosisLAA histology was available from 198 patients, of whom 38 (19.2%) had a previous catheter ablation. A previous failed catheter ablation was associated with increased collagen fiber density (fibers/mm2) (OR = 1.18; 95%CI, 1.01-1.39; P=.036). Collagen fiber area (2.3 [2.1-2.7] vs 2.3 [1.9-2.6] μm2; P=.70), fiber length (3.2 [3.0-3.4] vs 3.2 [2.9-3.4] μm; P=.95) and fiber diameter (0.77 [0.72-0.82] vs 0.76 [0.71-0.80] μm; P=.53) were equally distributed between patients with and without a previous catheter ablation, respectively. figure 2 shows the difference in the distribution of collagen fiber thickness. Both patient groups showed a bell-shaped density curve after log-transformation. Of these distributions, the median values were the same (figure 2A), but the width of the bell differed slightly but significantly (P <.001). This difference in distribution is shown in figure 2B.
DISCUSSIONPropensity matched analysisIn this propensity matched analysis, we found an increased risk of AF recurrence after thoracoscopic ablation in patients with a previous failed catheter ablation compared with ablation naïve controls, although most patients in the 2 groups did not experience AF recurrence during the 1-year follow-up. Both propensity score-based analyses (ie, propensity score matching with replacement of controls and conditional logistic regression) indicated an increased risk of AF recurrence for patients with a previous failed catheter ablation, after controlling for the included covariables. With our retrospective study design, we identified a previous failed catheter ablation as a marker of reduced efficacy. The estimated risk of recurrence was highest in the propensity matched analysis with replacement of controls, where each case was matched to a highly similar control. Inclusion of all eligible patients in the logistic regression model resulted in a more nuanced but significantly increased risk of AF recurrence. In this propensity matched selection of patients, the absolute freedom from AF rates may not reflect those of previously published cohort studies.4,6,8 Due to heterogeneous patients and treatment characteristics, there was sufficient overlap to perform these propensity score-based analyses. Small differences remained between the 2 matched groups. However, patients with a failed catheter ablation had more paroxysmal AF, fewer had a history of myocardial infarction, and fewer were aged ≥ 75 years, which may have reduced the differential risk of recurrence. Double adjustment of the primary analysis for all suboptimally balanced variables validated the results with a slightly lower, significant effect. We therefore argue that a satisfactory matching balance was achieved, taking into account the relatively low number of patients included and large number of variables included in calculation of the propensity score.
Clinical risk factors for atrial fibrillation recurrenceAt baseline, patients with a previous failed catheter ablation had a smaller LAVI, smaller right atrial volume index, less congestive heart failure and less persistent AF, which are all known predictors for recurrent AF. Other clinical risk factors for AF recurrence, including age, hypertension and female sex, did not differ between groups. An increased risk of AF recurrence, despite there being fewer risk factors for AF recurrence at baseline, appears contradictory. However, these risk factors may need a different interpretation in the setting of a previous catheter ablation, which is a marker for a worse prognosis. Perhaps existing risk factors for recurrence need to be interpreted differently in the presence of a failed catheter ablation. The left atrium may shrink following catheter ablation, irrespective of restoration of sinus rhythm14,15 and greater left atrial shrinkage has been described with more extensive ablation strategies.16 Ablation of atrial tissue may limit the possibility of sustained AF by reducing the total atrial surface17 and thereby possibly limiting AF to paroxysms rather than persistent episodes. Aside from obscured or reversed risk factors, the number of asymptomatic episodes may increase after initial catheter ablation,18 which may contribute to increased atrial remodeling with fewer or no symptoms. Last, the perception of symptoms may be reduced irrespective of the clinical success of the procedure,19 which may increase the symptom threshold before a repeat procedure is considered by the patient and physician. The proportion of AF and AT in both groups did not differ (data not shown).
To summarize, regardless of the presence of the usual predictors of AF recurrence, patients with a previous failed catheter ablation are more prone to failure of the thoracoscopic procedure. A failed catheter ablation may therefore be a marker of worse prognosis. At this point, we cannot exclude nor confirm that a failed catheter ablation mechanistically contributes to increased AF recurrence after thoracoscopic ablation. One or more catheter ablations may induce structural and/or electrical remodeling, which could make patients more susceptible to AF recurrence despite thoracoscopic ablation. Ablation lesions created during earlier catheter ablations may have become a source of ectopy or local conduction slowing. Moreover, incomplete, nontransmural, interrupted, or reconnected lines could give rise to re-entry circuits leading to atrial tachycardia.20 However, the current study was performed without endocardial mapping of the left atrial substrate. Hence, we were not informed about the transmurality of previous ablation lesions. We were unable to adjust for any previous catheter ablation lesions as these procedures were mainly performed in centers other than where the thoracoscopic ablation was performed. We cannot confirm nor exclude that ablation on previously ablated, mature scar tissue is less effective, and negatively affects the transmurality of new ablation lesions. The clinical success of repeat catheter ablations and periprocedural epicardial PVI on previously ablated pulmonary veins suggests that ablation on older ablation scars is feasible and effective, although data are lacking.
A plausible explanation for the difference in AF recurrence between these groups is that there is one (or multiple) hidden risk factor(s) at play, for which a failed catheter ablation here acts as a marker. For the primary analysis, no direct measures of the left atrial fibrotic substrate were available. Patients with a previous catheter ablation may have a more advanced form of AF, with a more progressed atrial substrate, for example due to a longer history of AF. Patients may have experienced more episodes of AF and thereby suffered from more AF-induced atrial remodeling.21 It would be expected that a more progressed atrial substrate would translate into more, or more severe, clinical characteristics of remodeling. However, we did not find a signal pointing to the presence of more established risk factors of AF recurrence. Conversely, if anything, patients with a previous failed catheter ablation seemed to have fewer clinical risk factors, apart from a longer history of AF.
To the best of our knowledge, there are only 3 studies reporting on the association between a failed catheter ablation and worse outcome after thoracoscopic ablation. Driessen et al.6 were the first to report this association in patients undergoing thoracoscopic ablation, during a 5-year follow-up. In the same center, but with different patients, Wesselink et al.13 found that a previous failed catheter ablation was an independent risk factor for AF recurrence during a 2-year follow-up in 121 participants with persistent AF. Last, Lim et al.7 found no association between a previous catheter ablation and freedom from AF. However, their ablation naïve patients (n=47) were older, had more persistent AF and larger LAVI, which may have decreased any differences in outcome with regard to patients with a failed catheter ablation.
There were, however, subtle differences in the organization of left atrial fibrosis between patients with and without a previous catheter ablation in the subset of patients in whom detailed histological analysis was available. Here, we show for the first time that collagen fiber density is increased in patients with a previous catheter ablation compared with ablation naïve patients, whereas the total percentage of collagen was unchanged. Moreover, the distribution of fibrosis fiber thickness had a different characteristic pattern between these patient groups, which was illustrated by the differential curve (figure 2B). This may be compatible with subclinical substrate progression, which is not (yet) reflected by clinical or electrophysiological baseline characteristics.
This subclinical substrate progression may explain the increased risk of AF recurrence. It is unknown whether these subtle differences affect conduction velocity or heterogeneity within the atrium and whether they may thereby contribute to arrhythmogenesis. Thick collagen fibers may decrease transversal conduction velocity, increase anisotropy and thereby increase the risk of (recurrent) AF.22 In the same way, increased density of collagen fibers may decrease conduction velocity.23 In turn, decreased conduction velocity increases the likelihood of re-entry and the risk of (recurrent) AF.24 Collagen fiber distribution and organization can thereby contribute to AF recurrence and may (in part) explain the increased risk of AF recurrence in patients with a previous failed catheter ablation.
LimitationsThis study is a retrospective analysis of patients undergoing thoracoscopic AF ablation. Although most of the data were prospectively collected, there are inherent limitations to a retrospective design, including confinement to associative conclusions, and the risk of selection or information bias. We aimed to minimize the risk of selection bias with a propensity matched design. In this propensity matched analysis, we were unable to correct for variables that were not observed or not structurally registered, and cannot speculate on the potential effect on the outcome of our study. We were unable to correct for characteristics of the failed catheter ablation(s), for example the applied lesion set, which may have affected the risk of recurrence. These data were not all available, as these procedures were mainly performed in other centers than where the thoracoscopic ablation was performed. We performed regular 24 hour Holter monitoring, which is a systematic limitation, but a limitation nonetheless. More intensive monitoring may have increased the detection of asymptomatic recurrences of AF; however, we have no reason to expect this effect to differ between patients with and without a previous failed catheter ablation. Due to intermittent monitoring, we were not able to report on the burden of AF in cases of recurrence.
CONCLUSIONSPatients with a prior failed catheter ablation undergoing thoracoscopic AF ablation have a considerably higher risk of AF recurrence than ablation naïve patients. This is despite a similar or even lower number of established clinically relevant risk factors for AF recurrence. A failed catheter ablation can be considered a marker for increased risk of recurrence. In parallel, we demonstrate that patients with a failed catheter ablation have more progressed subclinical atrial fibrosis formation.
FUNDINGThe authors received no financial support for the research, authorship, or publication of this article.
AUTHORS’ CONTRIBUTIONSR. Wesselink, W.J.P. van Boven, A.H.G. Driessen, and J.R. de Groot conceptualized and designed this project. R. Wesselink, M. Vroomen, I. Overeinder, J. Neefs, N.W.E. van den Berg, E.R. Meulendijks, F.R. Piersma, J. Luermans, B. Maesen, C. de Asmundis, G-B. Chierchia, M. La Meir, and L.A.F.G. Pison contributed to inclusion of participants and data acquisition. R. Wesselink, M. Vroomen, I. Overeinder, J. Neefs, N.W.E. van den Berg, E.R. Meulendijks, F.R. Piersma, R.F.M. Al-Shama, T.A.C. de Vries, T.E. Verstraelen, and J.R. de Groot participated in data analysis and interpretation. All authors contributed to drafting, critically appraising, and/or revising the manuscript. All authors approved the final version of the manuscript and are accountable for all aspects of the work. R. Wesselink and J.R. de Groot were responsible for the overall content of the manuscript.
CONFLICTS OF INTERESTB. Maesen is consultant for Atricure and Medtronic, J. Luermans has a consultancy agreement with Medtronic, G.B. Chierchia: AF solutions Medtronic, Biosense Webster, Abbott Medical, Boston Scientific, Philips, Biotronik, A.H.G. Driessen: Consultant for Atricure, last 5 years no revenue, L.A.F.G. Pison: consultant for Medtronic, M. La Meir: Consultant Atricure. J.R. de Groot is supported by research grants through his institution from Abbot, Atricure, Bayer, Boston Scientific, Daiichi Sankyo, Johnson & Johnson and Medtronic and received speaker fees/consulting fees from Atrian Medical, Atricure, Bayer, Biotronik, Daiichi Sankyo, IPPMed, Medtronic, Novartis and Servier. The other authors report no disclosures.
.
We cordially thank Prof Dr A.H. Zwinderman for his statistical support.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.09.006