Sprint Fidelis defibrillation leads are prone to early failure. Most of the reported series come from a single institution. This paper describes the clinical experience in nine Spanish hospitals.
MethodsClinical, implant, and follow-up data of all patients with a Sprint Fidelis lead were analyzed. All cases of lead failure were identified, medium-term lead survival was calculated, and possible predictors for lead failure were determined.
ResultsIn total, 378 leads in 376 patients were studied. The mean age (male 85.7%) was 64.9±13.6years. The majority of patients (59.8%) had ischemic heart disease. Mean left ventricular ejection fraction was 33.4%±14.5%. Left subclavian vein puncture was used in 74.8%. During a mean follow-up of 30.9±14 months, 16 lead failures have occurred, with a lead survival of 96.1% at 36 months after implantation. Eleven of 16 lead failures were caused by failure of pace/sense conductors, 3 by defects in the high-voltage conductor, and 2 by defects in both types of conductors. A less depressed left ventricular ejection fraction was associated with an increased probability of lead failure (42.4%±16% vs. 33%±14.3%; P=.011). Three hospitals presented a rate of lead failure higher than 10%; the rate was less than 5% in the remaining 6 hospitals.
ConclusionsIn this multicenter series of 378 leads, the 3-year estimated survival was higher than that reported in prior series. Clinical presentation of lead failures was similar to that reported previously. Left ventricular ejection fraction and hospital of implantation were variables associated to lead failure.
Keywords
The publication of several multicenter, international, randomized, controlled trials has shown the beneficial effect of implantable cardioverter device (ICD) in terms of decreased mortality in certain groups of patient at risk, both in a secondary and primary prevention setting.1 The increase in the number of indications has lead to an exponential growth in the number of implantation procedures.2 On the other hand, ICD implantation is associated with a series of potential complications during both the procedure and the follow-up. These complications include infection, generator decubitus, bruising, lead displacement, cardiac perforation, decreased quality of life due to shocks, proarrhythmia, defibrillation or pacing failure, inappropriate shocks for supraventricular arrhythmias, and oversensing.3 Some of these complications are due to failure of the defibrillator lead, and their non-negligible incidence has been demonstrated by data from series with long-term follow-up of different types of lead.4,5,6 Technical advances have not only focused on correcting problems with previous lead types but have also allowed smaller generators and narrower leads to be developed, in the expectation that this would reduce the number of complications associated with the size of the system. However, general experience has been different and small-diameter leads have been associated with higher complication rates.6,7 One of these new small-diameter leads is the Sprint Fidelis lead (Medtronic, Minneapolis, Minnesota, United States). The Food and Drug Administration approved the 6.6 Fr (2.2mm) Sprint Fidelis lead in the United States in September 2004, but they were withdrawn from the market in October 2007 due to a higher than expected fracture rate8; 268 000 leads are thought to have been implanted throughout the world. Different international series, mostly from single centers, have reported the clinical presentation and estimated the incidence of lead failure and possible risk factors, although results have not been consistent,8,9,10,11,12,13,14 particularly with regard to the incidence of lead failure. In the present study, we report general experience with Sprint Fidelis leads in nine Spanish centers.
Methods PatientsAll patients who received a Sprint Fidelis lead (6930, 6931, 6948, or 6949) were included. General demographic data such as age and sex were collected, as well as data on the indication for implantation, ventricular function, access route for the lead, subtype of Sprint Fidelis lead implanted, presence of appropriate and inappropriate shocks during follow-up, and time from implantation until the appearance of lead failure (if it occurred) or the last recorded follow-up visit. The number of Sprint Fidelis leads was recorded for data analysis, such that if a patient received more than one of these leads for whatever reason, the general characteristics were recorded again. The data on implantation were analyzed for the whole sample. The follow-up data were derived from recorded interviews and technical reports, including the interview prior to implantation and the interview when lead failure occurred. All cases of lead failure were identified and the data on the form of clinical presentation and subsequent approach once the problem had been identified were compiled. Lead failure was classified according to whether the pace/sense (P/S) conductor or the high-voltage lead had failed. Overall survival of the Spring Fidelisis leads in this series was calculated, and the data were analyzed for the overall sample and by each center.
DefinitionsFailure due to lead fracture, in any of the following circumstances:
– Inappropriate shocks resulting from oversensing of “noise” from nonphysiological signals.
– Sudden change in the chronic impedance for pacing or defibrillation (>20% over a 24-h period).10
The SPSS (version 16.0) software was used for statistical analysis (SPSS Inc., Chicago, Illinois, United States). Categorical variables were expressed as absolute numbers and percentages; quantitative variables were expressed as means (SD) and interquartile ranges. Categorical variables were compared using the Fisher test or the χ2 test; continuous variables were compared using the Student t test. The mean number of lead failures per year was calculated from the mean number of fractured leads observed during follow-up divided by the number of leads, multiplied by the mean duration of follow-up. The survival analysis was performed using the Kaplan-Meier technique. P-values<.05 were considered statistically significant.
ResultsBetween January 2005 and October 2007, the 9 participating centers implanted 378 Spint Fidelis leads in 376 patients. Only 2 models of Sprint Fidelis leads were used: the active fixation model 6949 (231 leads, 61.1%) and the passive fixation model 6948 (147 leads, 38.9%). The most frequently used venous access route for implantation of the Sprint Fidelis lead was direct puncture of the left subclavian vein (74.8%), followed by dissection of the left cephalic vein (19.1%), and direct puncture of the right subclavian vein (5.2%). The total number of leads implanted in each center is shown in Figure 1. The demographic characteristics of the patients studied can be seen in Table 1. Of note is that most were men (85.7%) with a mean age close to 65years and low left ventricular ejection fraction (LVEF), a median of 33.4% (14.45%). Ischemic heart disease was the main indication for implantation of ICDs, both in primary and secondary prevention (226 patients, 59.8%), followed by nonischemic dilated cardiomyopathy (100 patients, 26.5%). The mean follow-up was 30.9 (14) months (median, 33 [24-41] months). During follow-up, only 23% of the patients (87 subjects) received appropriate shocks from the device and 10.3% (39 patients) received inappropriate shocks, essentially due to supraventricular arrhythmias (71.1%).
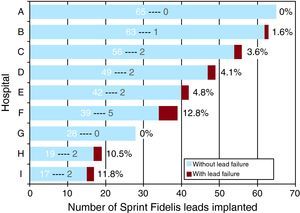
Figure 1. Number of Sprint Fidelis leads implanted in each center (white number), indicating the number of failed leads (grey) and the percentage of all leads implanted in each center.
Table 1. General Characteristics of the Patients and Comparison Between the Groups of Patients With and Without Lead Failure.
| Total patients (n=378) | With failure (n=16) | Without failure (n=362) | P | |
| Men, % | 324 (85.7) | 13 (81.3) | 311 (85.9) | .602 |
| Age, years | 64.9±13.6 | 61.4±16.4 | 65±13.5 | .297 |
| LVEF, % | 33.4±14.45 | 42.4±16 | 33±14.3 | .011 |
| Follow-up, months | 30.9±14 | 1140±446.5 | 964.3±415.4 | .109 |
| Indication for implantation | ||||
| Secondary ischemic heart disease | 115 (30.4) | 4 (25) | 111 (30.7) | .014 |
| Primary ischemic heart disease | 111 (29.4) | 4 (25) | 107 (29.6) | |
| Secondary nonischemic dilated cardiomyopathy | 46 (12.2) | 1 (6.3) | 45 (12.4) | |
| Primary nonischemic dilated cardiomyopathy | 54 (14.3) | 2 (12.5) | 52 (14.4) | |
| Hypertrophic cardiomyopathy | 15 (4) | 2 (12.5) | 13 (3.6) | |
| Brugada syndrome | 13 (3.4) | 1 (6.3) | 12 (3.3) | |
| Long QT syndrome | 6 (1.6) | 2 (12.5) | 4 (1.1) | |
| Others | 18 (4.8) | 0 | 18 (5) | |
| Access route (left subclavian) | 283 (74.8) | 14 (87.5) | 269 (74.1) | .775 |
| Sprint Fidelis model (6949) | 231 (61.1) | 12 (75) | 219 (60.5) | .127 |
| Appropriate shocks | 87 (23) | 3 (18.8) | 84 (23.2) | .679 |
| Inappropriate shocks | 39 (10.3) | 8 (50) | 31 (8.6) | <.001 |
LVEF, left ventricular ejection fraction; SD, standard deviation.
Data are expressed as mean±SD or n (%).
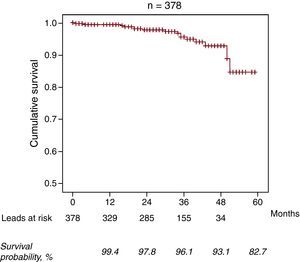
In total, 16 lead failures were reported (4.2%). The mean time to failure was 29.1 (14.9) months (range, 1.5-52 months; median 32 [20-38.5] months). The mean failure rate was 0.016 failures/lead-year. Table 2 summarizes the cases individually. The distribution of cases of lead failure by center is shown in Figure 1. Of note is the fact that the 3 centers with the highest number of leads implanted are among the 4 with the lowest rate of lead failure. In addition, most of the cases of lead failure (9 leads, 56.2%) are found in 3 centers, with an individual incidence of failure greater than 10%. Of the 16 cases of failed leads, most (13) occurred in the P/S conductor, with isolated failure being the most common (11 cases, 68.75%), whereas isolated failure of the high-voltage lead occurred in 3 leads (18.75%). All cases of failure of the high-voltage lead corresponded to model 6949 leads. In 2 patients with Sprint Fidelis lead failure, a new Sprint Fidelis lead was implanted because the procedure was performed prior to issuance of the safety warning. The percentage of Sprint Fidelis leads that survived the first year of follow-up was 99.4% (95% confidence interval [CI], 98.7%-100%). Of those that survived the first year, the survival rate between 12 and 24 months was 98.4% (95% CI, 96.9%-99.8%). Of those that survived 2years, the probability of survival for an additional year was 98.2% (95% CI, 96%-100%). Of those that survived 3years, the probability of survival for an additional year was 96.9% (95% CI, 93.4%-100%); at 4years survival the percentage with an additional year survival was 88.9% (95% CI, 75.3%-100%). Thus, the estimated cumulative survival at 3 and 4years was 96.1% and 93.1%, respectively (Figure 2).
Table 2. Description of Cases of Sprint Fidelis Lead Failure.
| Case | Hospital | Age (years) | Sex | Indication | LVEF (%) | Access route | Model | Follow-up (months) | Presentation | Type of failure | Approach taken | Review of complications |
| 1 | H | 74 | Male | Primary ischemia | 25 | Left subclavian | 6949 | 35.5 | Warnings | High energy | Removal+implantation different DL | No |
| 2 | H | 57 | Male | Primary ischemia | 30 | Left subclavian | 6949 | 50.5 | Inappropriate shocks | P/S | Removal+implantation different DL | No |
| 3 | C | 49 | Male | Secondary ischemia | 30 | Left cephalic | 6949 | 17.9 | Inappropriate shocks | P/S | Withdrawal+implantation Sprint Fidelis | No |
| 4 | C | 65 | Male | Secondary hypertrophic cardiomyopathy | 55 | Left cephalic | 6949 | 43.5 | Inappropriate shocks | P/S | Removal+implantation different DL | No |
| 5 | E | 63 | Female | Primary nonischemic dilated | 28 | Left subclavian | 6949 | 1.5 | Regular follow-up | P/S | Lead was maintained for defibrillation and pacing with LV lead | — |
| 6 | E | 59 | Male | Secondary ischemia | 55 | Left subclavian | 6949 | 30.7 | Inappropriate shocks | P/S | Removal+implantation different DL | No |
| 7 | I | 61 | Male | Brugada syndrome | 65 | Left subclavian | 6948 | 34 | Warnings | P/S | Removal+implantation different DL | No |
| 8 | I | 74 | Male | Primary nonischemic dilated | 25 | Left subclavian | 6948 | 37.8 | Inappropriate shocks | P/S | Removal+implantation different DL | No |
| 9 | F | 79 | Male | Secondary ischemia | 35 | Left subclavian | 6949 | 52 | Inappropriate shocks | P/S | Implantation P/S lead | Loss LV capture |
| 10 | F | 50 | Female | Long QT syndrome | 60 | Left subclavian | 6949 | 35.8 | Inappropriate shocks | P/S | Removal+implantation different DL | No |
| 11 | F | 73 | Male | Primary ischemia | 60 | Left subclavian | 6949 | 4.5 | Inappropriate shocks | High energy and P/S | Withdrawal+implantation Sprint Fidelis | No |
| 12 | F | 82 | Male | Secondary hypertrophic cardiomyopathy | 45 | Left subclavian | 6949 | 16.6 | Inappropriate shocks | High energy and P/S | Removal+implantation different DL | Late infection |
| 13 | F | 13 | Male | Long QT syndrome | 70 | Left subclavian | 6949 | 20.7 | Warnings | High energy | Removal+implantation different DL | No |
| 14 | B | 63 | Female | Secondary ischemia | 40 | Left subclavian | 6948 | 40.3 | Regular follow-up | P/S | Removal+implantation different DL | No |
| 15 | D | 51 | Male | Secondary nonischemic dilated | 31 | Left subclavian | 6949 | 23.6 | Warnings | P/S | Removal+implantation different DL | No |
| 16 | D | 69 | Male | Primary ischemia | 25 | Left subclavian | 6949 | 20.8 | Warnings | High energy | Removal+implantation different DL | No |
DL, defibrillation lead; LV, left ventricle; LVEF, left ventricular ejection fraction; P/S, pace/sense.
Figure 2. Survival curve for the Sprint Fidelis leads.
The main form of clinical presentation of failures in the P/S conductor (9 out of 13 patients, 69.2%) was inappropriate shocks due to excess sensitivity to nonphysiological signals, accompanied by sudden prior increases in the pacing impedance (Figure 3). Three cases of failure of the P/S conductor occurred in patients with the lead integrity alert (LIA) algorithm activated (cases 2, 7, and 15) (Table 2). The algorithm prevented inappropriate shocks in 2 of these patients but not the other because of failure to recognize the activated acoustic warning signal given days before the shocks were received (case 2). In the remaining cases, the problem of failure occurred without having the algorithm activated and the conventional alerts were not effective at preventing inappropriate shocks. In contrast, the acoustic warnings by the device were the form of presentation when failure occurred in isolation in the high-voltage lead.
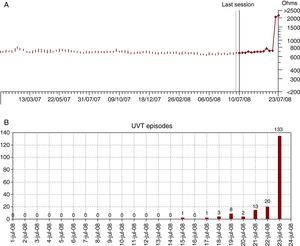
Figure 3. Plot of trends in impedance to pacing (A) and episodes of unsustained ventricular tachycardia (B) in a case (patient 8) with failure of the pace/sense conductor that presented in the form of an electrical storm with more than 30 shocks. The sudden increase in impedance to pacing above 2000Ω on the day prior to clinical presentation is evident, along with the number of UVTs for oversensing 1 day after the increase in the impedance to pacing. UVT, unsustained ventricular tachycardia.
In 15 of the 16 cases of lead failure, the approach after diagnosis was surgical revision, with the failed lead abandoned and a new defibrillation lead implanted in most cases (Table 2), without any serious acute complications associated with the procedure in any case. At 9 months after surgical revision, 1 patient (case 12) presented an infection of the new lead that became complicated, despite extraction, with bacterial endocarditis leading to death.
Comparison of the general characteristics between patients with lead failure and those without failure is shown in Table 1. Age, sex, and duration of follow-up were not statistically associated with lead failure. Likewise, access route for implantation and the Sprint Fidelis model did not result in significant differences between the 2 groups. In the group of patients with lead failure, hypertrophic cardiomyopathy, Brugada syndrome, long QT syndrome, and other less common indications were more frequent, although the groups were similar with respect to the more common indications. Patients with Sprint Fidelis lead failure had a significantly lower LVEF (42.4% vs 33%; P=.011). In contrast, patient comparisons between centers with a failure rate >10% and those with a rate <10% did not show any significant differences in terms of ventricular function. However, there was a greater frequency of left cephalic approach (23% vs 6.8%; P=.01) and a shorter follow-up time (29.8 months vs 35.2 months; P=.002) in the overall group of patients from centers with a failure rate less than 10%; in addition, model 6948 showed a higher failure rate (41.6% vs 27.4%; P=.026).
DiscussionIn this multicenter study that analyzed all Sprint Fidelis leads implanted in 9 hospitals, a 3-year survival for the lead of 96.1% and a 4-year survival of 93.1% were estimated. The most common clinical presentation was presence of inappropriate shocks in relation to nonphysiological oversensing due to fractures in the P/S part of the lead.
Survival of the Sprint Fidelis LeadsThe estimated survival at 3years after implantation of the Sprint Fidelis lead was significantly greater than that reported in the main previous series derived from studies of 1 or 2 centers (90.8%11; 87.9%13; 89.3% at 30 months15). However, our figures are close to those provided by Medtronic in their survival reports from the System Longevity Study and CareLink, with 3-year survivals of 95% and 97%, respectively.16,17 The causes of these discrepancies are likely to be multifactorial and not readily identifiable. Possibilities include differences in the sample size, participating centers, and data collection method. Although some authors have suggested that the studies not sponsored by Medtronic would have a closer follow-up and greater rigor in identifying problem cases, and so their data would be more reliable than those of the manufacturer,18 this hypothesis does not apply in our study and would not explain the differences observed.
The factors that influence lead failure are varied and include the inherent characteristics of the lead, the access route, the operator, and individual patient characteristics. In the case of Sprint Fidelis leads, repeated comparison with other leads of similar characteristics implanted in the same years reflected a greater failure rate, confirming the influence of factors inherent to the configuration and structure of the lead and its greater vulnerability to fracture.11,13
Clinical PresentationThe main form of clinical presentation in cases of Sprint Fidelis lead failure was inappropriate shocks due to oversensing caused by “noise” from fracture of the P/S conductor (9 patients) (Figure 4). In only 2 cases of fracture of the P/S conductor was the problem detected as a result of prior acoustic warnings programmed in the device. One of these standard warnings, the one activated by increased impedance, was ineffective for reducing inappropriate shocks in cases of Sprint Fidelis lead failure.19 In our series, the increases in pacing impedance were not preventative in any case. However, in Spain, most of the previously implanted devices had access, beginning in September 2009, to a new algorithm, the LIA, (which was incorporated in the newer models), that encompassed oversensing and increased impedance parameters. Although not perfect, it has been shown to reduce inappropriate shocks in these patients by allowing early preclinical detection of lead failure.20,21,22 This was the case in 2 of the 3 patients in whom P/S conductor failure occurred with the LIA activated.
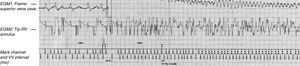
Figure 4. Example of inappropriate shock and proarrhythmia in a patient of the series. On the left, intracardiac electrograms (top: shock electrogram; bottom: bipolar electrogram) in atrial fibrillation during which there is a continuous oversensing of “noise” arising from sensing in the area of ventricular fibrillation. This produces a high-voltage shock that triggers a true episode of ventricular fibrillation.
Variables Associated With FailureIn the 16 cases of Sprint Fidelis lead failure, better ventricular function was associated with a greater rate of lead failure. This association is in agreement with that reported in several other series.10,12 In the series reported by Farwell et al.,10 the authors observed that a better LVEF and venous access not involving cephalic dissection were independent predictors of failure. In that study, each 10% increase in LVEF increased the risk of fracture 2.42 times. Better ventricular function could be associated with greater stress of the electrode tip in each cardiac cycle due to a more energetic contraction, thereby explaining the association. Thus, younger age has also been proposed as a variable that might influence fracture, as the patients will potentially be more physically active.15 In the series that we present, we did not find any association between lead failure and older or younger age of the patients. Likewise, in other series, there was no association between age and risk of fracture,10,12 and it can be stated in general that the vigor of the cardiac contraction and the resulting stress of the lead is more closely related to LVEF (lower value, less lead stress) than the degree of activity in this patient population, most of whom have decreased LVEF.
In our study, we observed significant differences in the failure rate between centers, such that 3 centers with a variable number of implantations exceeded 10% incidence of lead failure. Specifically, one of the centers accounted for 5 of the 16 failures reported. These data are not comparable with those published for a previous multicenter trial in which no statistically significant differences between center or operators were observed, although the incidence of the problem varied between 0 and 3.2%.14 Although in the centers with greater incidence of failure, noncephalic access was more frequent than in the other centers, in 14 of the 16 cases of failure, the access route was left subclavian puncture, which made analysis of this variable difficult. It is possible that other factors, such as the manipulation of the lead itself by the operator or the duration of the implant, might play some role in the differences observed.23
Lead Longevity and Risk of FailureAnother interesting aspect of this study is that the risk of failure increases with the life of the lead and is not high in the periprocedural phase. This suggests that the fracture does not occur, at least completely, during implantation and that patient factors and the access route might have an influence in each individual patient. In the initial study that raised the alert about the early failure of these leads,8 there were no long-term data, but subsequent series have confirmed that the risk of fracture and its incidence increase over time. In the 2-center study with a large number of implantations of these leads, Hauser et al.13 observed that the failure rate of the Sprint Fidelis leads was 3.75% at 1 year and that the total for other different lead models was 0.58%. The estimated 3-year survival from implantation of the Sprint Fidelis leads was 87.9%, which contrasts with the 95% calculated for the other group of leads. These data are in line with other subsequent studies.10,11 This fact points to an increasing risk of lead failure in patients implanted with a Sprint Fidelis lead, who should therefore be monitored closely. In our series, although the estimated 3-year survival is greater than in previous studies, a noteworthy decrease is observed for longer follow-up periods (Figure 2).
Dealing With Failed and Nonfailed LeadsAlthough systematic replacement of leads in all patients with a normal-functioning Sprint Fidelis lead does not appear to be advisable, as the risk of surgical revision is considered to be higher than the risk of lead failure,24 some authors suggest this possibility in certain patient populations with a greater potential risk, such as those affected by ion channel disorders or hypertrophic cardiomyopathy.13 In our series of patients with lead failure, the approach of the investigators was variable, with abandonment or extraction of the lead and implantation of a new defibrillation lead the most common. No more than 1 serious complication arose in the surgical revision (6.25%), although this case had a fatal outcome. The data in the literature indicate that abandonment of the leads is safer than their extraction,24 and abandonment seems to be a safe option.25
LimitationsThe main limitations of the present study include its retrospective character and the number of patients studied, as the relatively low incidence of the problem is associated with a low number of cases of lead failure. However, data from a number of centers could be collected to allow the role of the implantation center and its operators to be assessed. The study did not compare the results of the Sprint Fidelis leads with those of other similar models of leads implanted by the same centers in a similar period, although, as reflected in the study by Hauser et al.,13 it is not expected that the centers with most failures of Sprint Fidelis leads also have higher rates of failure of other lead models. The small number of failed leads removed is not enough to provide data on the site and mode of fracture of the lead. The follow-up period is short, and so this analysis cannot provide information on the change in risk of failure beyond the period observed.
ConclusionsIn this multicenter series of 378 Sprint Fidelis defibrillation leads, the estimated 3-year survival from implantation was greater than reported in other previous series in the literature. Our results, however, confirm that survival is clearly time-dependent. Patients should therefore be closely monitored and the alert programs should be implemented in these patients to allow early detection and minimize the risk of inappropriate shocks and other problems related to lead failure. The clinical presentation of cases of failure was similar to that reported previously, and left ventricular function and the implantation center were variables related to lead failure.
Conflicts of interestNone declared.
Received 20 September 2010
Accepted 20 November 2010
Corresponding author: Unidad de Arritmias y Electrofisiología Cardiaca, Hospital Virgen de la Salud, Avda. Barber 30, 45004 Toledo, Spain. maapalomares@secardiologia.es