INTRODUCTION
The regular practice of moderate, aerobic exercise is associated with a healthy plasma lipid profile1 and a reduced risk of coronary artery disease and cardiovascular-related death.2,3 The results of cross-sectional4,5 and longitudinal6 studies suggest that aerobic exercise increases plasma levels of high density lipoprotein cholesterol (HDL-C) and reduces triglycerides and low density lipoprotein cholesterol (LDL-C) levels. However, contradictory results have been reported with respect to certain types of exercise demanding strenuous physical exertion,7-9 especially in terms of lipoprotein (a) [Lp(a)] levels.10 Several studies report no differences in plasma Lp(a) levels between physically trained and sedentary individuals,11-13 whereas others report higher levels in trained subjects.1,14 Contradictory results have also been obtained in experimental studies.12,13,15 There are several possible explanations for these discrepancies, including differences in the methods used to determine the levels of lipids and lipoproteins, the difference in physical fitness of the individuals tested, ethnic factors, or differences in training history or the type, quantity and intensity of exercise undertaken, etc. On their own, increases in Lp(a) as a consequence of high intensity or stressful exercise are given little clinical importance. However, when both Lp(a) and LDL-C levels are high, the risk of cardiovascular disease increases exponentially.10 Lp(a) has been identified as an atherogenic and thrombogenic factor16 that can act synergistically with other cardiovascular risk factors.17-19 In fact, the study of the interactions between apolipoprotein B100 (apo B100), LDL-C and Lp(a) is of interest in the characterization of cardiovascular risk.11,19
The aims of the present work were: a) to compare the plasma lipid profile of persons who practice different types of sport involving different levels of physical stress, and b) to characterize the possible interactions between the most important variables of the plasma lipid profile, especially those involving Lp(a).
SUBJECTS AND METHODS
Three groups of sportsmen--swimmers (n=29), volleyball players (n=17), and soccer players (n=23)--were studied, all of whom competed at the regional/national level. For comparison, a fourth, control group was established, composed of healthy, sedentary individuals (n=26) comparable to the sportsmen in terms of age, sex and anthropometric and sociological characteristics. Table 1 summarizes the characteristics of the study subjects. All subjects gave their consent to be included in the study after being informed of its characteristics and scientific interest, and all completed a questionnaire on their health and food habits. All subjects were healthy, took no medication or herbal products that might influence the lipid profile results, and were free of intercurrent disease. All consumed a typical, Spanish Mediterranean diet;20 no significant qualitative differences were recorded between the groups. The subjects were informed of the importance of not embarking on any excessive dietary practices and of not imbibing excessive amounts of alcohol (2 drinks/day maximum, ≤30 g alcohol/day) in the 2 weeks before the taking of blood samples. They were also told not to undertake any stressful physical exertion in the preceding 48 hours.
Blood samples were taken after a 12 hour nocturnal fast. Standard biochemical and hematological profiles were determined as well as plasma lipid profiles. A number of blood samples were taken at different times during the year; no seasonal differences were seen in the results.
Training History of the Study Subjects
All subjects completed a questionnaire on their physical activity over the last 5 years. Their trainers provided information on the characteristics of the training undertaken (quantity, intensity, number of sessions per day and week) and the frequency of competition.
The 28 swimmers selected had been registered for 10±4.4 years and took part in regional and national competitions. The total time spent in training and competition was 13.4±2.1 h per week. Training was divided into 90% aerobic resistance and 10% elasticity and joint movement. At the beginning of the season, muscular strength was developed using rubber tubing. Table 2 shows the exercise characteristics of the sport.
The 17 volleyball players selected had been registered for 9.4±1.5 years and played in the national premier division. At the time of the study they trained once per day for 5 days per week, and played a match on the weekend. The total time spent in training and competition was 15.3±2.2 h per week. Training included ~55% intermittent high intensity training with prolonged recovery times between series; the remaining time was given over to the development of muscular strength through specific explosive force training (mainly for the legs). Table 2 shows the exercise characteristics of the sport.
The 23 soccer players selected competed in the second division of the national professional league and had practiced this sport for the last 14.5±3.1 years. At the time of the study they trained once per day, 4 days per week, but on Wednesdays they had a double training session. They also played 1 or 2 matches per week (weekends and during the week). The time spent training or in matches was 19.3±2.5 h per week. Training included ~60% intermittent high intensity training with short recovery times between series; the remaining time was given over to the development of muscular strength through specific explosive force training. Physical contact and falls, etc were common during training and matches--a natural feature of the game. Table 2 shows the exercise characteristics of the sport.
The training and competition characteristics recorded (Table 2) show the swimmers to be subject to a less stressful type of exercise. The training and competition characteristics endured by the soccer players, plus the emotional and environmental factors surrounding this sport,21 show these sportsmen undergo more stressful exercise regimens. Training characteristics were considered to be an ordinal variable.
Study of Plasma Lipid Profiles
Plasma total cholesterol (TC), LDL-C, and HDL-C levels were determined by an enzymatic colorimetric method using a Hitachi 911 autoanalyzer (Roche Diagnostics; Indianapolis, USA). Apolipoprotein A-I, apo B100, and Lp(a) levels were determined by nephelometric kinetics (Array 306, Beckman Coulter® Inc.; Fullerton, USA). Since Lp(a) values can change depending on the length of time serum samples are refrigerated,12 samples were left for no longer than 4 hours at ambient temperature and were refrigerated (2-8 °C) for no longer than 4 days.
Assessment of Lp(a) Interactions
Subjects were grouped into 4 categories depending on their plasma Lp(a) and LDL-C levels (cut-off values 32 mg/dL and 128 mg/dL respectively). They were also grouped into 4 other categories depending on their plasma Lp(a) (cut-off value 32 mg/dL) and TC/HDL-C levels (the cut-off value corresponded to the third quartile value of the control subjects). Two interactions were therefore analyzed: that between Lp(a) and LDL-C, and that between Lp(a) and TC/HDL-C. Finally, an additional category was established to include subjects with high levels of Lp(a), LDL-C, and TC/HDL-C.
Assessment of Apo B100 Interactions
Subjects were grouped into 4 categories depending on their plasma apo B100 (the cut-off value corresponded to the third quartile value of the control subjects) and Lp(a) levels (cut-off value 32 mg/dL). They were also grouped into 4 other categories depending on their plasma apo B100 (cut-off value=third quartile value of the control subjects) and plasma LDL-C levels (cut-off 128 mg/dL). Two interactions were therefore analyzed: that between apo B100 and Lp(a), and that between apo B100 and LDL-C. An additional category was established to include subjects with high apo B100, Lp(a), and LDL-C levels.
Statistical Analysis
Normality was tested using the Shapiro-Wilks test. The Kruskal-Wallis test was used to compare non-normally distributed variables. One way analysis of variance (ANOVA) was used to compare normally distributed variables. To compare the homogeneity of the variance, the Levenne test was used. When variances were homogeneous, comparisons were made using the post hoc Tukey test; the post hoc Games-Howell test was used for non-homogenous variances. Descriptive values for normally distributed variables are represented as means±standard deviation (SD); non-normally distributed variables with an asymmetry of <0.5 or >0.5 are represented as geometric means±SD. The odds ratio (OR) for each sport group was calculated using the Mantel-Haenszel method, taking the swimmers, soccer players, and volleyball players to be exposed groups, and the sedentary controls to be non-exposed. Cardiovascular risk factors were considered high when indicators were above the established cut-off points: Lp(a), 32 mg/dL and LDL-C, 128 mg/dL.17-19 For the remaining variables, the cut-off points were established as the third quartile of the sedentary control values, except for apo A-I and HDL-C, the cut-off values for which were fixed at the first quartile. The test for trends was used to determine the relationship between the type of physical exercise and cardiovascular risk factors. Significance was set at α=0.05 in all cases, except for the Shapiro-Wilks test, in which it was set at α=0.20. Ninety five percent confidence limits were determined.
RESULTS
Table 1 shows the characteristics of the participating subjects. Owing to the selection criteria, the groups showed no significant differences except with respect to their exercise regimens. Table 2 shows that the degree of muscular overload or physical stress was greatest for the soccer players and least for the swimmers.
Tables 3 and 4 respectively show the lipid profiles and atherogenic indices of the 3 groups of sportsmen and the control group. Though the lipid profiles of the different groups were within the reference range for the subjects' age, and sex, they show significant differences. The plasma lipid and lipoprotein levels (Table 3) and the atherogenic indices (Table 4) of the swimmers were significantly healthier than those of the soccer and volleyball players. The soccer players had higher plasma levels of Lp(a) than any other group, including the control group (Table 3). The swimmers had the highest apo A-I values; their apo B100 and LDL-C levels were, however, lower than those of the soccer or volleyball players (Table 3). The atherogenic indices showed similar patterns.
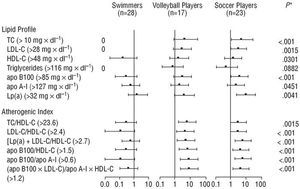
The Figure shows the probability--the OR--of having an abnormal lipid profile (always compared to the control group). This analysis clearly shows the swimmers to have healthier lipid profiles, whereas soccer players and volleyball players were at a clear disadvantage, i.e., they had a particularly high probability of their plasma lipid profile being negatively affected. Therefore, the greater the physical stress endured, the greater the risk of having a lipid profile defined as a cardiovascular risk.
Table 5 shows the interactions of Lp(a) and apo B100 with other lipid cardiovascular risk factors. The swimmers showed no tendency towards having high Lp(a) or apo B100 levels associated with high LDL-C or TC/HDL-C levels. In contrast, the volleyball players showed a clear tendency to have high Lp(a) and apo B100 levels associated with high LDL-C and TC/HDL-C levels--a tendency even stronger among the soccer players.
In addition, both the volleyball and the soccer players had a significantly lower OR for having simultaneously low plasma Lp(a) and LDL-C, Lp(a), and TC/HDL-C, or Lp(a) and apo B100 levels. Therefore, the test for trends shows that the OR for having abnormalities with respect to the interactions of lipid profile components is directly related to the increase in stressful physical exertion (Table 5 and Figure 1).
Fig. 1. Odds ratio of having a high lipid cardiovascular risk factor in the indicated groups. The black squares are the odds ratio values; the error bars express the 95% CI. Apo indicates apolipoprotein; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TC, total cholesterol; Lp(a), lipoprotein (a). *P value obtained with the test for trends.
DISCUSSION
The sportsmen subject to the highest levels of physical stress were the soccer players; these showed negative alterations in their atherogenic indices. The interactions of Lp(a) and apo B100 with other cardiovascular risk factors confirmed the possible impairment of the lipid profile among these sportsmen. In addition, these abnormalities were directly and significantly associated with the degree of physical stress to which the different groups of sportsmen were subject. The values for HDL-C, LDL-C13,22,23, apo A-I22,23, and Lp(a)24-27 recorded in earlier studies agreed with the present results. To our knowledge, this is the first study to analyze the interactions of Lp(a) and apo B100 with other cardiovascular risk factors.
It is difficult to offer an explanation of why the most stressful exercise should impair the lipid profile. However, it is well known that intense exercise, particular-ly eccentric exercise accompanied by falls and collisions,28 leads to the release of proinflammatory cytokines29-31 associated with abnormalities in metabolism.32,33 In addition, the endotoxin (lipopolysaccharide [LPS]) of the cell walls of Gram-negative bacteria could be involved in the inflammatory response of several cellular disorders. The quantities of circulating LPS in healthy subjects are inappreciable, but they increase after strenuous exercise.34 It has also been shown that LPS can impair lipid metabolism, increasing the activity of 3-hydroxymethylglutaryl coenzyme A reductase and reducing the level and activity of cholesterol 7α-hydrolase mRNA.35,36
Given the near or total absence of eccentric contractions, contact or falls in swimming, the acute cytokine, and endotoxin responses are probably reduced. Any negative changes occasioned by these molecules with respect to lipid metabolism are therefore avoided. In addition, the aerobic training practiced by the swimmers might be responsible for the healthy effects observed in their lipid profiles.
Atherosclerosis is a progressive disease characterized by the accumulation of lipids and fibrous elements in the major arteries. Its etiology is difficult to define but the risk factors include a poor lipid profile, increased fluid shear stress,37 and increased susceptibility of the lipoproteins to oxidation.38,39 Accordingly, the negative lipid profiles of some of the subjects in this and other studies8,10,40 might be counteracted by adaptations to physical exercise, especially improved antioxidant and fibrinolytic capacities and reduced fluid shear stress.41-43 This suggests the cut-off values for lipids associated with cardiovascular risk should be raised in sportspersons who compete at national level. Further studies are needed to corroborate this hypothesis. At present, no evidence exists to suggest that sportsmen subject to high levels of stressful physical exertion (e.g., soccer or rugby players) are at any greater risk of ischemic heart disease.
The present results take on great importance for occasional practitioners of strenuous sports (whose aim it is to improve their physical fitness on weekends43) involving eccentric contractions, physical contact and falls (e.g., those who play soccer, rugby or basketball). Given the poorer physical fitness characterizing this sector of the population, their antioxidant and fibrinolytic activities and the fluid shear stress they experience may not be optimized. In such persons, the negative lipid profile caused by stressful physical exertion might not be counteracted by protective factors, leading to an increased risk of atherothrombogenesis. Further studies are necessary to corroborate this hypothesis.
CONCLUSIONS
In conclusion, the practice of sports involving a high degree of stressful exertion, such as soccer and volleyball, is accompanied by unfavorable plasma lipid and lipoprotein profiles. In contrast, sports with low levels of stressful exercise, such as swimming, appear to have a favorable effect on plasma lipid profiles.
Full English text available at: www.revespcardiol.org
See Editorial on Pages 495-8
ABBREVIATIONS
TC: total cholesterol.
LDL-C: low density lipoprotein cholesterol.
HDL-C: high density lipoprotein cholesterol.
apo A-I: apolipoprotein A-I.
apo B100: apolipoprotein B100.
Lp(a): lipoprotein (a).
Correspondence: Dr. J. Ruiz Ruiz.
Departamento de Fisiología. Facultad de Medicina.
Universidad de Granada.
Avda. de Madrid, s/n. 18012 Granada. España.
E-mail: ruizj@ugr.es