To the Editor,
Aortic stenosis is the most frequent valvular heart disease in the developed world.1 The natural history of severe symptomatic aortic stenosis (SSAS) offers little hope of survival and valve substitution surgery is the only effective treatment.2 However, 30%-40% of patients with SSAS are not operated as they present too high an estimated surgical risk.3 Survival and quality of life are limited in these patients. Percutaneous balloon valvuloplasty was initially considered an alternative therapy but it offers very poor mid-term results.4 Recently, percutaneous implantation of prosthetic aortic valves (PIAP) has been introduced as a valid option for patients with SSAS and prohibitive surgical risk.5-8
One contraindication of PIAP is severe interventricular septal hypertrophy because the prosthesis may be displaced and migrate during implantation. We describe a simple modification to aortic prosthesis deployment that has enabled us to treat PIAP successfully (Edwards-Sapien prosthetic valve) in 2 patients with inoperable SSAS and severe interventricular septal hypertrophy.
Two patients with SSAS (men, aged 79 and 90 years) were referred for PIAP with Edwards-Sapien prostheses (Edwards Lifesciences Inc, Irvine, California, US). The patient selection process8,9 revealed no contraindication for the technique other than severe interventricular septal hypertrophy (18 mm and 19 mm, respectively). We decided to proceed with the intervention.
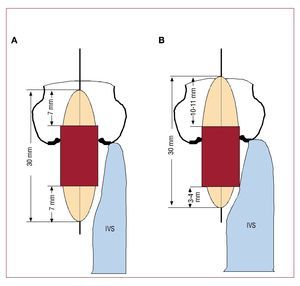
In both patients, we implanted an 26 mm Edwards-Sapien prosthesis, an expandable aortic valve balloon prosthesis delivered via a stainless steel stent, with 3 bovine pericardial valves. It is assembled in the interventional cardiology laboratory with a 30 mm long balloon. The 26 mm valve is 16 mm long, and its location in the middle of the balloon is recommended, leaving 7 mm of balloon on either side of the prosthesis. Severe interventricular septal hypertrophy is considered a contraindication for PIAP because when balloon inflation—and therefore, implantation—takes place, the prosthesis may be displaced by the septum and migrate towards the ascending aorta. In these 2 patients, we minimized contact between the balloon and the interventricular septum by positioning the prosthetic valve asymmetrically, more distal, leaving only 3-4 mm of balloon on the intraventricular side of the prosthesis (Figure). Thus, we were able to implant the prostheses successfully in both 2 patients, without displacement, with optimal expansion, and without periprosthetic insufficiency. Both patients were discharged without complications.
Figure 1.A: deployment of the prosthesis in a patient without severe interventricular septal hypertrophy. The prosthesis is positioned symmetrically in the balloon. B: patient with severe interventricular septal hypertrophy. To minimize contact between the balloon and the interventricular septum, the valve is located in the balloon asymmetrically, more distal, to leave only 3-4 mm of the balloon on the ventricular side of the prosthesis.
These 2 patients described show severe interventricular septal hypertrophy, not necessarily a contraindication for transcatheter aortic valve implantation (TAVI) of Edwards-Sapien prostheses. This technique can only be recommended for patients with severe interventricular septal hypertrophy, given that the risk of prosthesis displacement in most patients is very low when the prosthesis is located symmetrically in the balloon.