Keywords
INTRODUCTION
Implantation of temporary epicardial electrodes in cardiac surgery is common practice. Generally, the ventricular electrodes are placed in the right ventricle (RV). It has not been confirmed that this is the best site for pacing. RV pacing can resolve the arrhythmic complications, but it does not induce physiological activation of the left ventricle (LV), and initiates electrical activation and asynchronous ventricular contraction.
Different studies have shown the potential negative effect of RV pacing, particularly when performed over long time periods, as reflected by an increase in the number of hospitalizations and a decrease in the functional class of patients, essentially in those with ventricular failure.1-5
Tissue Doppler (TD) imaging techniques can allow an analysis of mechanical asynchrony.6,7 The myocardial strain analysis used in this study has the advantage of differentiating between active myocardial contraction and passive myocardial movement, and it has been used to demonstrate mechanical asynchrony.7-9
Placement of electrodes in the LV using the coronary sinus as access can be very difficult, as it depends on the venous anatomy. However, in cardiac surgery, electrodes can be placed in any epicardial position and the effect of pacing at each of these sites can be observed.
Therefore, we have performed this study in patients submitted to cardiac surgery, in whom temporary electrodes were implanted in the RV and in different epicardial positions in the LV. The objectives of the study were, on one hand, to analyze the effects of acute pacing in the RV and at different sites in the LV on synchrony, and on the other, to determine whether there was a site where greatest heart efficiency could be obtained after pacing.
METHODS
Study Population
Consecutive adult patients were selected from a waiting list for elective cardiac surgery. These patients were in sinus rhythm and gave their written informed consent to participate in the study, which was approved by the ethics committee of our hospital. Exclusion criteria were atrial fibrillation, complete left bundle-branch block, implanted pacemaker, and failure to meet the inclusion criteria. Nineteen patients were included (4 women [21%]) with mean (SD) age of 68 (10.62) years. The mean (SD) ejection fraction (EF), estimated from echocardiography using the Simpson method10 was 57.6% (14.6%). Only 4 patients had an ejection fraction <50 actual values 25 30 45 and 16 patients had ischemic heart disease 6 a prior infarction 4 anteroapical 2 inferobasal all were in sinus rhythm no qrs interval duration greater than 120 ms the corresponds to part of electrocardiogram that represents ventricular polarization sixteen underwent revascularization 3 aortic valve replacement serious postoperative complications reported p
Surgical Technique
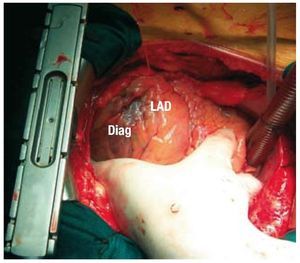
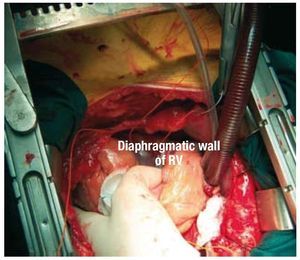
Temporary epicardial electrodes were implanted at 3 locations in the LV (anterior, inferior, and lateral), as well as in the inferior segment of the RV close to the apex (Figures 1 and 2). Temporary atrial electrodes and 2 skin electrodes were also implanted as indifferent electrodes.
Figure 1. Temporary electrode in the anterior segment of the left electrode, between the left anterior descending artery (LAD) and the diagonal artery (Diag).
Figure 2. Temporary electrode placed in the diaphragmatic wall of the right ventricle (RV).
External generators were used (5388 dual-chamber Medtronic, Medtronic Inc., Minneapolis, Minnesota, USA). The atrial electrodes were connected to the atrial channel of generator no. 1 in bipolar mode. Pacing of different ventricular regions was done in monopolar mode using 1 of the skin electrodes as the positive electrode. The pacing frequency was 10 bmp greater than the heart rate of the patient and the atrioventricular interval was 130 ms. These parameters remained fixed regardless of the region paced. Ventricular capture during pacing was confirmed by QRS morphological analysis of the surface electrocardiogram (ECG). The electrodes were withdrawn after finishing the study.
Echocardiographic Study
The echocardiographic study was performed between the third and fifth day after surgery. A Vivid 7 device (General Electrics Medical systems, Horton, Norway) was used with a 3.4 MHz probe.
Five consecutive echocardiographic studies were undertaken; without pacing, with RV pacing, with pacing at the anterior wall, with lateral pacing, and with pacing in the lower LV.
Images were obtained using parasternal projections in the long and short axes and apical planes in 2, 3, and 4 chambers. The LV outlet flow velocity was recorded in the 5-chamber apical plane using pulsed Doppler technique. Five consecutive beats were obtained in each plane during the baseline study and during each pacing.
Asynchrony was analyzed using 2-dimensional images obtained with TD techniques. The studies were performed in the 2-, 3-, and 4-chamber apical planes. To achieve a higher frame rate, the narrowest sector that permitted satisfactory analysis of the ventricular walls, excluding the atrial walls was used. The frame rate was always >100 fps. Five consecutive beats were obtained in each plane. The images were recorded with the patients holding their breaths, with optimal ECG signal, and excluding extrasystoles.
Analysis of the Echocardiographic Studies
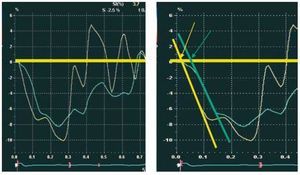
The data were collected using an external work station (Echopac, General Electrics, Horton, Norway). Different measurements in mode M, 2 dimensions, and the determination of EF were carried out in accordance with the recommendations of the American Society of Echocardiography using the Simpson method.10 Cardiac output (CO) was determined by Doppler echocardiography.11 To obtain the strain curves (e), a region of interest 10x30 mm was defined in the basal and medial regions of each of the 6 segments of the LV (anterior, anterolateral, inferolateral, inferior, inferoseptal, and anteroseptal), and in the lateral wall of the RV. For each segment, the time was measured from onset of the QRS complex to the where the line that follows the descending fundamental part of the systole curve exceeded zero velocity. The time at which the line crossed the line of zero velocity was used to measure the onset of strain (Figure 3), in similar fashion to that published previously for cardiac magnetic resonance imaging (MRI) studies.12 The average time from 5 cardiac cycles were used. To obtain synchrony indexes, we used the mean time of each of the ventricular segments (mean of baseline and medial segments).
Figure 3. For each segment, the strain-time curves were measured from the onset of the QRS complex where the line that follows the descending fundamental part of the systole curve exceeded zero velocity (arrows). In yellow, the septal segment curve, and in green, the lateral segment.
Asynchrony Parameters
To estimate the interventricular mechanical asynchrony, the difference in time to onset of strain between the RV and LV was used (Te R/L), that is, the mean time from the onset of strain in the 6 segments of the LV.
To estimate intraventricular asynchrony, the following measures were used: SD of the time of start of strain of the 6 segments of the LV (Te SD), and maximum difference between the time from start of myocardial strain of 2 different segments of the LV (Te MD).
Statistical Analysis
Quantitative data were expressed as means (SD). The correlation between the different quantitative parameters was analyzed using the Spearman test. The Friedman and Wilcoxon tests were used for analysis of differences between paired groups, using the Bonferroni correction. The intraclass correlation coefficient (ICC) and 95% confidence intervals (CI) were used to evaluate the intraobserver and interobserver agreement in the analysis of Te SD, Te MD, and calculation of the integral of flow velocity at the LV outlet tract. To estimate the variability in the calculation of CO, this variable was analyzed, given that it is the only one that is modified when CO is calculated with the different types of pacing. Statistical significance was set at P<.05. SPSS (version 11.0) software was used for the statistical analysis (SPSS Inc., Chicago, Illinois, USA).
RESULTS
The mean (SD) asynchrony parameters were Te LV, 28.3 (56.9) ms; Te SD, 36.6 (34.9) ms; and Te MD, 90.5 (87.4) ms. There was a good correlation between the last 2 parameters (r=0.971; P<.0001).
Likewise, Te SD and Te MD showed a good correlation with EF (r=0.62 and r=0.6, respectively; P<.001) and with a history of prior infarction. Patients with prior infarction and low EF had worse asynchrony parameters. Patients with prior infarction (n=6) showed greater asynchrony, but without reaching statistical significance: Te R/ L, 13.9 (45.9) versus 28.19 (24.8) ms; Te SD, 23.7 (11.3) versus 41.9 (25.2) ms (P=.06); Te MD, 60 (25.9) versus 100 (67.2) ms (P=.1).
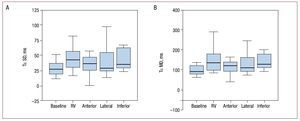
All asynchrony parameters increased with respect to baseline values when the RV was paced: Te R/L, 59.8 (40.5) versus 28.23 (56.9) ms (P=.002); Te SD, 53.2 (34.4) versus 36.6 (34.9) ms (P=.007); Te MD, 135.3 (82.9) versus 90.5 (87.4) ms (P=.007) (Table; Figures 4 and 5). This pacing was the one that produced most asynchrony (Table). Pacing in the anterior and lateral segments of the LV lead to less asynchrony than pacing in the RV. Likewise, pacing in the anterior region of the LV produced less difference with respect to the baseline study in the asynchrony parameters analyzed (Table).
Figure. 4. Bar diagram representing the difference in time between the onset of strain in the right and left ventricle (Tε R/L) at baseline and after each type of pacing. RV indicates right ventricle.
Figure 5. Bar diagram. A: SD in time from onset of strain in the 6 segments of the left ventricle (Tε SD). B: maximum difference in the time of onset of strain in the 6 segments of the left ventricle (Tε MD), at baseline and after pacing at different sites. RV indicates right ventricle.
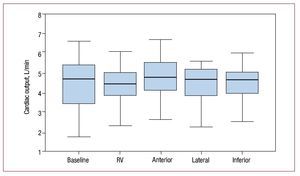
The mean (SD) CO obtained in the different studies was as follows: baseline, 4.4 (1.2); RV pacing; 4.3 (1), pacing in the anterior segment of the LV, 4.7 (1); pacing in the lateral segment of the LV, 4.5 (1.2); pacing in the inferior segment, 4.4 (0.9) (Friedman test, P=.0018). The CO after pacing at the anterior segment of the LV was significantly greater than that obtained after RV pacing (Wilcoxon test, P=.001).
The remaining types of pacing at other sites in the LV did not show statistical differences in the CO with respect to pacing in the RV (Figure 6).
Figure 6. Bar diagram representing cardiac output at baseline and after pacing at the different sites. RV indicates right ventricle.
The interobserver and intraobserver ICCs were 0.74 (0.48-0.88) and 0.89 (0.77-0.95) for Te SD and 0.73 (0.46-0.87) and 0.87 (0.73-0.94) for Te MD, respectively. The interobserver and intraobserver ICCs for estimating the integral of the LV outlet tract velocity were 0.997 (0.992-0.999) and 0.998 (0.996-0.999), respectively.
DISCUSSION
This is the first study to analyze in detail the acute effect on mechanical asynchrony and efficacy of cardiac contractile function after pacing at different sites during the period immediately after heart surgery. Our study shows that, of the different pacing sites analyzed, the RV is the site that produces most interventricular and intraventricular asynchrony, whereas the sites that give a result most similar to baseline are the anterior and lateral segment of the LV. On the other hand, although the CO obtained with pacing at these sites of the LV is not different to that obtained in baseline conditions, pacing at the anterior segment of the LV yields significantly greater COs than those obtained when the pacing site is the RV.
Method Validity
The utility of studying mechanical synchrony is subject to debate, essentially after publication of the PROSPECT study,13 in which the mechanical asynchrony indexes did not appear to be reliable indicators of response after resynchronization therapy. However, asynchrony was not determined using strain-based methods, and so the results cannot be extrapolated to studies performed using these methods. Recent studies using the strain technique have shown greater utility at predicting the effect of resynchronization therapy.14-16 A recent study compared the velocity-based methods and strain-based methods obtained with TD techniques to determine which parameter is the best predictor of response after resynchronization therapy.17 No parameter obtained with TD velocities indicated improvement after resynchronization therapy, whereas the SD of the strain was a good predictor of volume reduction after resynchronization.
We have analyzed asynchrony parameters based on strain in a similar fashion in a previous study,17 but here we used a broad sector, with simultaneous recording of opposing segments. We could thus avoid measurement errors caused by variation in cardiac frequency. However, the frame rate obtained is lower, and the segment alignment is not always ideal. These problems are not so great in our patients because there was a good signal-to-noise ratio and alignment was easier given they did not have dilated hearts. We also measured the strain time in a different way. Sometimes, it is difficult to select the time peak, either because there might be multiple peaks or because the peaks might be variable. To avoid this problem, for our study, we chose the point where the straight line that shows the main deformation of the segment exceeds zero strain. We thus reflect the main behavior of the strain curve and homogenize the measurement of extrapolating the curve at the same time, the zero value of strain. This approach avoids the difficulty of measurement associated with more than one strain peak or with poor definition of that peak (Figure 3). This method has been previously validated in cardiac MRI studies,12 and the variability study obtained good results in the ICCs for the different measures used.
Effect of Pacing on Cardiac Function in Patients With Impaired and Conserved Ventricular Function
The asynchrony caused by RV pacing gives rise to different deleterious effects, particularly in patients with low EF: decreased EF, increased episodes of heart failure, and increased mortality.18-20 In addition, in patients with ventricular dysfunction and conduction disorders, biventricular or LV pacing is more beneficial.21-28
Our study population was not characterized by impaired ventricular function. A recent study in patients with abnormal sinus rhythm who received a dual-chamber device and who had normal atrioventricular node conduction and EF has shown that RV pacing leads to a decrease in EF in the long-term.29 In addition, Puggioni et al,30 on comparing LV pacing with RV pacing in patients with atrial fibrillation and atrioventricular node ablation, found that LV pacing led to a favorable acute hemodynamic effect, with an increase in EF and a decrease in the degree of mitral valve failure. The effect was similar in patients with normal EF and impaired EF.
Similarly, our study shows that RV pacing is associated with worsening of the interventricular and intraventricular asynchrony parameters and that LV pacing causes less interventricular and intraventricular asynchrony than RV pacing. The pacing sites associated with least asynchrony were the anterior and lateral walls of the LV. This pacing was not accompanied by an increase in cardiac output, although greater output was observed with RV pacing.
Studies After Cardiac Surgery
Few studies are available that analyze the effect of different types of pacing in the postoperative period after cardiac surgery, and the studies that are available have reported contradictory results. Healy et al31 showed the DDD mode pacing (pacemaker with dual sensing, dual pacing, and dual response) increased coronary flow, CO, and flow from the coronary duct with respect to VVI mode pacing (left ventricular volume). Dual-chamber pacing did not have an additive effect. Likewise, Schmith et al32 did not find any improvement in the general or segmental left ventricular function parameters in patients with cardiac failure and conduction disorders when performing dual-chamber pacing after surgery. Bakhtiary et al,33 in contrast, observed an increase in CO with dual-chamber pacing in approximately 59% of patients with ventricular function impairment and broad QRS. Flynn et al34 studied patients with ventricular dysfunction. Using a thermodilution catheter, they analyzed the effect of pacing in the RV and anterior and posterior segment of the LV and showed that pacing at the inferolateral wall of the LV increased the cardiac index and mean arterial pressure compared to RV pacing. They concluded that pacing in the inferolateral segment of the LV provides a beneficial effect in surgical patients.
The aforementioned studies were performed in patients with LV dysfunction and generally with conduction disorders. However, the present study was conducted in patients with normal QRS who were not selected for impaired EF. In these patients, we found that the changes in CO achieved by changing pacing site are small. Nevertheless, we were able to observe a trend toward decreasing CO on RV pacing in contrast to pacing in the anterior segment of the LV.
Study Limitations
The patients studied were not selected for ventricular dysfunction or bundle-branch block. Therefore, the results cannot be extrapolated to other studies that recruited patients with left ventricular dysfunction or bundle-branch block. Given that the number of patients studied was small, the results should be confirmed in subsequent studies.
Different studies such as the PROSPECT trial13 have not confirmed the validity of the usual methods for assessing mechanical asynchrony and, in general, these methods are not very reproducible. In contrast, our method, on using the same patient as a baseline control, minimizes errors of interpretation. Moreover, these changes are easier to analyze than comparisons between 2 groups of patients. Analysis of radial and circumferential strain would have provided more information for the study and would have increased its validity. Unfortunately, the study was done using TD techniques, and so radial and circumferential analyses were impossible.
In this study, we did not analyze EF or ventricular volumes as parameters of ventricular function. To carry out these measurements reliably and reproducibly, good image quality is necessary, although this is not always easy to obtain after cardiac surgery. We therefore preferred to analyze changes in CO, which only depends on changes in the Doppler signal in the LV outlet tract and is less influenced by image quality.
CONCLUSIONS
RV pacing led to significant interventricular and intraventricular asynchrony in patients in the immediate postoperative period after cardiac surgery. In these patients, the pacing sites that led to least asynchrony were the lateral and anterior wall of the LV.
Although there were no significant differences in CO of the patients without pacing compared to those with pacing, we did see a significant difference between CO obtained with RV pacing compared to that obtained with pacing of the LV anterior wall.
In view of our results, we believe that the usual pacing site should be changed, given how easy it is to place electrodes on any part of the epicardium after surgery. If the patient does not have ventricular dysfunction, the electrodes should be placed in the atrium and the anterior or lateral segment of the LV for DDD mode pacing.
ACKNOWLEDGMENTS
We thank the members of the Department of Biostatistics, and Alfonso Muriel and V¨ªctor Abraira in particular.
ABBREVIATIONS
e: strain, myocardial deformation
Te MD: maximum difference in the time of onset of strain in the 6 segments of the left ventricle
Te R/L: difference in time to onset of strain between the right and left ventricle
Te SD: SD in time from onset of strain in the 6 segments of the left ventricle
Correspondence: Dr. J.L. Moya Mur.
Hospital Ram¨®n y Cajal.
Ctra. Colmenar, Km 9,300, 28034 Madrid. Spain.
E-mail: jmoya.hrc@salud.madrid.org
Received April 22, 2009.
Accepted for publication March 12, 2010.