Keywords
INTRODUCTION
Atrial fibrillation (AF) is the most frequent preoperative cardiac arrhythmia among patients undergoing heart surgery. In the subgroup of patients with mitral valve disease, the prevalence is much higher, between 40% and 80%.1,2 Among adult patients with atrial septal defect, the prevalence is 41%,3 and in ischemic heart disease, it is 0.6%.4 The prevalence is superior to 5.9% in patients over the age of 65 years undergoing surgery.5
The maze surgical procedure is currently the most effective treatment for AF, achieving cardioversion to sinus node rhythm in more than 90% of patients.6-9 However, because of its technical complexity, it has been performed in only a small number of patients. Various surgical groups have developed simpler and more rapid alternative procedures, but in most cases the results achieved have not equaled those of the maze procedure.10-12 Radiofrequency (RF) applied intraoperatively by electrocatheters has been shown to be able to reproduce maze surgical atriotomies in a rapid, safe, and effective way.13-16
This study reports the initial intraoperative experience of our group with RF energy applied to the epicardium and endocardium for biatrial compartmentalization in a group of patients with chronic AF requiring surgical repair of cardiac valve disease.
PATIENTS AND METHOD
Between June and November of 2000, 10 patients in chronic AF (>6 months) underwent surgery with extracorporeal circulation for valvular heart disease, with associated intraoperative RF for the treatment of their arrhythmia. The surgical procedure used in this study was biatrial compartmentalization created by RF applied with a surgical probe. The surgical indication was cardiac valve disease. All patients were informed about the procedure and gave their consent in writing. Patients with associated coronary disease were excluded. The study group was formed by 4 men and 6 women aged 51 to 69 years (60±11 years). The surgical heart disease was: mitral cardiac valve disease in 6 patients (rheumatic in 5 cases, degenerative in one), mitroaortic cardiac valve disease in 2 patients, aortic cardiac valve disease of degenerative origin in 1 patient, and incomplete atrioventricular canal in 1 patient. In two patients the intervention was a mitral reoperation. The antiquity of AF was documented electrocardiographically and ranged from 7 months to 14 years, mean 7.8±4.8 years. One patient had chronic AF of less than 3 years, and 9 had AF for more than 3 years. The electrocardiographic voltage of the f wave in precordial lead V1 was 0.074±0.08 mV, between 0.01 mV and 0.2 mV. Most of the patients had taken at least two different antiarrhythmic drugs to control AF, fundamentally digitalis and amiodarone; the average number of antiarrhythmic agents tried per patient was 1.9±0.3 (range, 1-3). One woman had a medical history of hypothyroidism secondary to amiodarone. At the time of the intervention, all patients consumed some type of antiarrhythmic. The main clinical data are summarized in Table 1.
Preoperative echocardiography (ECHO) and postoperative controls were carried out with a Hewlett-Packard Sonos 1500 Doppler echocardiograph. In each patient, the diameters of the left atrium were measured in millimeters, in addition to the usual echocardiographic parameters. The area of the left atrium was evaluated by echocardiographic planimetry in the apical four-chamber axis, and atrial volume was calculated by applying the formula of an ellipse.17 All measurements were made in end-systole. The atrial contribution to left and right ventricular filling was assessed by echo-Doppler, measuring the peak speed of atrial contraction (A wave) in m/s, speed of early diastolic atrial filling (E wave) in m/s, and the A/E ratio.
Intraoperative RF was applied using a malleable Thermaline® (Model 15.907, Boston Scientific Corporation, EP Technologies) surgical probe, with two null electrode plates located on the patient´s back. The surgical RF probe consisted of 7 10-mm long coil type electrodes, separated 3 mm, which allows long lesions of up to 9 cm to be made when the ablation is done simultaneously through all of them. Each electrode has two temperature sensors, located at its ends. The probe was connected to an RF generator (EPT 1000XP) through a connection box (EPT Meca APM 830T), which allows the electrodes to be used in each lesion to be selected based on the anatomic variants of the patient. The ablation protocol used was 100 W for 120 s, under temperature control, with an 85°C limit for atria of normal thickness and 75°C for thin-walled atria (<2 mm) in order to avoid excessive lesion with risk of perforation.16,18 The thickness of the atrial wall was evaluated grossly by the surgeon. Previous trials in animals helped us to prepare a specific ablation protocol for endocardial and epicardial applications, and to confirm its safety and effectiveness. In these tests we also obtained a better (more continuous) ablation line when RF was applied from the smooth surface of the right atrial epicardium, probably because better tissue-electrode contact was obtained, which was barely comparable to that reached through the endocardial trabeculated surface. In the surgical field, aside from controlling the temperature during ablation, the surgeon visual assessed the ablation line. When the ablation was grossly discontinuous in some point, it was repeated until a uniform line of white coagulation was achieved. All lesions were made in a blood-free field. Hearing «pops» was not a criterion for stopping the ablation. Visual inspection of the lesion made it possible to analyze other aspects characteristic of RF ablation, such as points of tissue carbonization. After each ablation, the lesion was cleaned manually with surgical gauze of small carbonization particles, which were originated mostly by contact with blood. The ablation was always made before implanting the cardiac prosthesis to avoid any anomalous lead in the current.
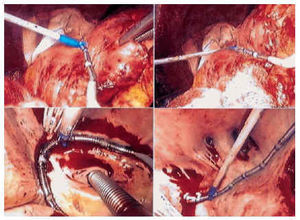
We chose to reproduce an approach similar to the maze III modification with the RF probe, with the changes described below (Figure 1). The heart was exposed by medial longitudinal sternotomy. Before initiating ablation for AF, sodium heparin was administered (3 mg/kg). The procedure began in the right atrium, without extracorporeal circulation. Epicardially, two independent ablation lines were created, the first along the crista terminalis from the superior cava to the inferior cava, and the second perpendicular to the first line, from the left lower pulmonary vein to the tricuspid annulus, along the right appendage (Figure 2). These two lesions were made in conditions of myocardial normothermia. Cryoablation did not involve the tricuspid annulus, or resection of the right appendage. Next, the patient was cannulated for extracorporeal circulation, inducing a moderate systemic hypothermia of 30°C. The heart was stopped with B/Braun® hematic cardioplegic solution (4°C; K=25 mEq/l; pH=7.6; hematocrit=20%) at 4°C by the anterograde and retrograde pathway. The hypothermia of the heart was maintained by topical lavage with cold saline solution (4°C). The left atrium was approached in the usual way, through the interatrial sulcus. All ablation lines in this atrium were made from the endocardium in myocardial hypothermia, at less than 25°C. The ostium of the left and right pulmonary veins were isolated independently by means of two circumferential lesions linked by another line along the posterior wall of atrium (Figure 2). The other two ablation lines connected the circumference of the left pulmonary veins with the mitral annulus and left appendage. The ablation lines next to the circumflex coronary artery were made simultaneously with retrograde perfusion via the coronary sinus of cold blood, at 4°C. Cryoablation was not performed in the point of the mitral annulus and the left appendage was not removed, although its was closed by internal suture. Maze III septal atriotomy was not reproduced in these patients. After finalizing the ablation, the scheduled surgical procedure was performed.
Fig. 1. Atrial pattern of ablation lines, represented with a broken line. LAp indicates left appendage; RAp, right appendage; SVC, superior vena cava; IVC, inferior vena cava; M, mitral; T, tricuspid.
Fig. 2. Upper photographs, right atrial epicardial ablation. Lower left photograph, endocardial isolation of the ostia of the left pulmonary veins; lower right photograph, isolation of the ostia of the right pulmonary veins. Note the number of ostia of the right pulmonary veins, specifically five.
Cardiac rhythm was monitored continuously during the first 48 h in the intensive care unit (Hewlett-Packard System Model 64S). Three temporary atrial epicardial electrodes (in the left atrial roof, sinus node zone, and anterior part of the free right atrial wall) and two ventricular electrodes were left in place in all patients for stimulation, recording cardiac rhythms, and/or atrial overstimulation (Myo/WireTM, size 2-0, temporary cardiac pacing wire, A&E Medical Corp., U.S.). Unipolar epicardial atriograms were used to diagnose and typify postoperative arrhythmias, grouping AF in 3 varieties:19 type A, defined as regular atriograms separated by isoelectric segments, with little fragmentation; type B, irregular atriograms with disturbances in the isoelectric line and/or marked fragmentation; type C, with alternation of types A and B. Paroxysmal AF was considered present when the arrhythmia occurred in episodes lasting less than 24 h, with intervals of regular atrial rhythm; and chronic AF when the episode lasted more than 24 h.
The antiarrhythmic treatment of the patient was not discontinued before the intervention. During surgery, the patient began treatment with amiodarone (300 mg/day i.v. bolus), which continued in the immediate postoperative period (1200 mg i.v. for the first 48 h) and later (200 oral mg/day). Postoperative arrhythmias were treated by cardioversion if they had hemodynamic effects. Antiarrhythmic prophylaxis with amiodarone was maintained for the first 60 postoperative days and later discontinued in the patients who remained in sinus rhythm. Other antiarrhythmic drugs like digoxin, calcium antagonists, and beta-blockers were added as needed for the patient´s condition. All patients received diuretic treatment with spironolactone, 50-100 mg/day during the hospital stay, to avoid water retention as a result of the decrease in natriuretic factor due to lesion and elimination of atrial tissue. Anticoagulation was discontinued the third month in patients without a mechanical cardiac prosthesis and with effective atrial contraction in the echocardiographic controls. Postoperative follow-up was carried out at discharge and three months later by electrocardiography and echocardiography. A Holter study was made only when the patient complained of new episodes of palpitations.
The data are expressed statistically as mean±standard deviation. This study is part of the clinical phase of the project financed by grant FISS 99/0484 for the study and development of intraoperative systems of RF in the treatment of AF.
RESULTS
In association with the surgical antiarrhythmic procedure, 6 replacements and 3 repairs were made on the mitral valve (2 plasties and one commissurotomy), one aortic valve substitution, and one closure of an ASD with mitral and tricuspid plasty. In one patient with a giant left atrium, the atrial size was normalized by tissue reduction. There was hospital or late mortality during the mean follow-up period of 3 months (range, 1-5.5 months). The mean cardiac ischemia time was 70±47 min (range, 38-114 min) and extracorporeal circulation lasted 109±47 min (range, 65-170 min). All patients were extubated in the first 48 h, except patient 3, who required prolonged intubation (for secondary, severe pulmonary hypertension that was later complicated by nosocomial pneumonia). The postoperative mean blood loss was 741±475 ml. No patient required reintervention for bleeding. Patient 4 presented a transitory cerebral ischemic accident on postoperative day 3. The mean hospital stay was 10.9 days (range, 7-26 days).
The lines of ablation in the right atrium were made with just 3 applications of the probe in approximately 11 min. In the left atrium the lines were made by 4 successive applications of the probe in approximately 16 min. During RF application, no injury involving atrial perforation occurred, although in some areas the wall thickness was minimal, less than 1 mm, particularly in the right atrium. In the epicardial ablations, frequent points of carbonization were appreciated at the ends of the electrodes, probably due to a border effect. In all the ablations, the evolution of the lesion could be controlled visually. In almost 11% of the applications, there were macroscopic gaps of healthy tissue, that is, a point in which the tissue discoloring characteristic of white RF coagulation was lacking. RF was again applied to these points. This circumstance is more frequent in endocardial lesions of the left atrium. The right atrial ablation lines could be made in all the patients before beginning extracorporeal circulation, thus avoiding a more prolonged extracorporeal circulation and myocardial ischemia time. These applications of RF to the right atrium did not in any case jeopardize the hemodynamics of the patient or produce any arrhythmic disturbance that forced interruption of the procedure. Also, RF did not produce any appreciable lesion in any neighboring organ.
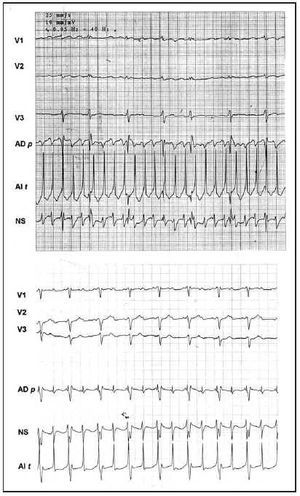
No patient recovered sinus rhythm simply with the application of RF to the right atrium. All patients left the operating room without AF. Electrocardiographically, in the first hours 6 patients presented a nodal escape rhythm that required temporary stimulation with a DDD pacemaker for the first 2.7 days (range, 1-5). The other 4 patients recovered sinus rhythm in the operating room and one presented bradycardia and needed pacemaker support (<45 bpm). During the rest of the hospital stay, 8 patients presented some type of arrhythmia: three of them had episodes of paroxysmal AF (case 1, type A with an approximate cycle length of 230 ms, case 5, type A and about 220 ms, and case 8, type A and about 210 ms), and the other three had first and/or second degree atrioventricular block, and one patient had atypical 2:1 flutter (320 ms) and two had sinus node dysfunction (Figure 3). No patient reinitiated chronic AF during the hospital stay. At the time of release, 9 (90%) patients had recovered sinus rhythm and 1 patient had persistent atrial flutter. In the 3 cases of recurrence of AF, the atriograms showed a regular pattern, with cycle lengths of more than 210 ms, that could be classified as atrial flutter or tachycardia. However, the surface ECG revealed a variability between beats of more than 30 ms in all of them, which is why we considered them as new cases of AF. A postoperative electrophysiological study could not be made in any of the 3 patients due to the transitory character of AF during the hospital stay. The patient with flutter was studied electrophysiologically during the postoperative period, confirming the presence of atypical flutter of left origin. Transeptal catheterization for ablation of the presumed gap was not performed, so the patient was discharged. During follow-up, the patient recovered sinus rhythm spontaneously.
Fig. 3. Unipolar epicardial atriograms. In the upper tracing (Case 1), regular type A fibrillation with a cycle length of 230 ms Lower tracing (Case 3), atrial flutter 2:1. Leads in the left atrial roof (LA r), free right atrial sinus node zone (SN), and free right atrial wall (RA w).
After follow-up, recurrence of chronic AF was documented in 2 patients (20%), in spite of pharmacological treatment and cardioversion. Eight patients (80%) recovered sinus rhythm, among them two who presented sinus dysfunction but did not have a pacemaker indication in the Holter study (Table 2).
Echocardiographic ally, at the end of follow-up left atrial contraction with a transmitral A wave was observed in only one patient. In contrast, none of the other patients in sinus rhythm showed effective recovery of left atrial contraction (zero t ransmitral A wave), although three of them presented an A wave of transtricuspid flow due to effective right atrial contraction (Table 2). In accordance with the Santa Cruz classification of Melo et al20 for the recovery of atrial contraction after AF surgery, one patient (10%) had a score of 4, three (30%) had 3, four (40%) had 1, and two (20%) had 0.
DISCUSSION
AF is the preoperative arrhythmia most frequently found in patients undergoing cardiac surgery. Between 10% and 15% present some variety of AF, in most cases chronic. In patients with surgical mitral cardiac valve disease, this incidence is greater, between 40% and 80%.1,2 Its clinical consequences are well known: loss of atrioventricular synchronism, a subjective sensation by the patient of irregular heart beat due to an irregular ventricular rhythm, hemodynamic deterioration as a result of the loss of atrial contractile function, risk of thromboembolism due to atrial blood stasis, onset of cardiomyopathy of arrhythmogenic origin, and decreased quality of life and life expectancy.
At present, it is uncommon to treat AF surgically, in part due to the complexity and morbidity per se of the surgical techniques. Various groups have tried to simplify this type of surgery and have described modifications in the maze III technique: the Kosakai-maze described by the author in 1994,21 atrial compartmentalization by Shyu et al in 1994,11 the left atrial maze by Sueda et al in 1996,22 modified maze by RF by Beukema et al in 1998,23 isolation of the pulmonary veins by RF by Melo et al in 1999,24 and partial atriectomy.25-27 All of them are examples of this surgical modality. However, although these procedures manage to restore sinus rhythm, the results are generally inferior to those of the maze procedure.7,8
In recent years, RF electrocatheters are being perfected for the purpose of reproducing surgical maze atriotomy by transmural thermal ablation lines.28-31 Beukema et al in 1998 communicated their initial experience in 60 patients who underwent surgery for mitral valve disease to which RF ablation of AF by means of maze III was added. A success rate of 80% was achieved after one year of follow-up.23 Several groups, including our own, have initiated our experience using RF for AF during the treatment of mitral valve disease. A partial maze made in the left atrium (isolation of pulmonary veins, the left atrial appendage, and mitral annulus) has yielded an initial success rate ranging from 50% to 77%.15,16,32 For this procedure we used long multielectrode probes with which to perform the lesions with the smallest possible number of applications and surgical time. Other groups have used RF differently and have achieved satisfactory results in 60% to 82% of patients; they prefer unielectrode surgical probes of shorter length, use different left atrial ablation line patterns, bipolar ablation, ect.15,33,34 Overall, these studies have validated intraoperative RF as a safe surgical technique with minimal associated morbidity, capable of creating atrial lesions rapidly, which makes it possible to shorten the duration of myocardial ischemia and extracorporeal circulation. Nevertheless, it is difficult to attain the results of the surgical maze procedure, which has almost 99% success, with RF.7,8 The explanation of these less satisfactory results could be attributed to various factors, notably scant intraoperative experience with the use of RF, the use of different ablation line patterns, mainly circumscribed to the left atrium (left maze) and, perhaps, to the fact that the design of the surgical probes is still under development. There are still aspects of intraoperative RF that remain to be defined, such as the energy protocols, the modality of unipolar or bipolar ablation, the endocardial and/or epicardial approach, geometry and design of the surgical electrode, ablation in normothermia or myocardial hypothermia, patterns of atrial lesion based on the cardiac disease, ect. More intraoperative studies are probably necessary if we wish to attain results comparable to those of surgical maze with RF.
At present, there is little clinical experience with the surgical treatment of AF by maze III (right and left atrial maze) using RF.23,35 Most groups limit the use of RF to a partial left maze procedure in patients with mitral valve disease. Our experience with RF in the treatment of AF also began with the left maze procedure, but we are now using the complete maze III pattern of ablation lines. We prefer biatrial ablation lines for several reasons. In the first place, we think that in AF associated with mitral disease the maze biatrial pattern of lesions is necessary to improve the initial results reached with RF applied according to a maze left pattern. After reviewing the bibliography, doubts remain as to which is the surgical procedure of choice for the treatment of AF in patients with mitral valve disease. The best results have been published with the maze III procedure, with a success rate better than 88% and almost 99% long-term effectiveness.7-9,36,37 The studies of Harada et al39 and Sueda et al22 have demonstrated recovery of the sinus rhythm in 74% with the left maze procedure in mitral valve disease.38,39 Kosakai et al found no differences between the two modalities of the maze technique, maze III and partial left atrial maze, when applied to the treatment of AF with mitral valve disease, although the number of recurrences during follow-up was greater with the partial procedure.40 Another reason why our group prefers to use biatrial ablation lines is the possibility of surgically treating AF associated with nonmitral disease, that is to say, associated with aortic valve disease, interatrial septal defect and/or ischemic heart disease. In these patients, there are more unified criteria in favor of the complete maze procedure, that is, AF is always treated with the maze III procedure because the results have been better than with partial maze procedures.7,40,41
Our pattern of atrial ablation lines did not correspond exactly with that of maze. These modifications are based exclusively on surgical reasons conditioned by our setting. We did not use cryoablation because we did not have the equipment and we did not make a lesion in the atrial septum to avoid opening the right atrium. In an attempt to simplify to the number of ablation lines and, consequently, the procedure, we made lesions in the right atrial free wall with a single ablation, thus including lesions of the right appendage. Like other authors, we do not think that it is necessary to perform elimination appendectomy of the right atrial appendage, although we do perform appendicular lesion-partition.16,42,43 In contrast, atrial appendectomy could cause postoperative problems of fluid retention in the third space because of the decrease in natriuretic factor, which has been observed by other groups and us with the reduction of atrial tissue.27,44-46 Some surgical teams do not perform cryoablation on the atrial annulus or septostomy, but they still obtain good results.13,33,47,48 The other left ablation lines that we make correspond to those performed by teams working with radiofrequency. Complete isolation and the combined isolated of the right and left pulmonary veins carried out in the maze procedure is equivalent to exclusion of 30-35% of the atrial tissue mass, a percentage that may affect the recovery of atrial contraction.42,49 At present, we prefer to isolate the right and left pulmonary veins selectively, then link them with an ablation line. In spite of the changes introduced by each author, no studies have been made to compare these modifications of the maze technique.
Epicardial ablation has been described in animal models and later applied to humans by isolation of the pulmonary veins for the treatment of AF secondary to mitral valve disease.16,32,50 There is little experience with surgical epicardial ablation in the right atrium.18 In our cases, epicardial ablation was simple, rapid, and safe (no perforation), even in the cases in which it was performed on thin-walled atria.
In our experience, at points of difficult access in the atria, particularly the left atrium, discontinuous lesions occur for different reasons: the lack of contact between the electrode and endocardium, differences in contact pressures between electrodes, or, sometimes, a surgical field with remains of blood. The RF source did not have sufficient sensitivity to detect these differences in the contact of the electrodes against the tissue. Macroscopically, the lesions presented gaps in 11%, especially the points corresponding to the central portion of each electrode. Visual control of the lesion was required to guarantee that it had a uniform appearance, without discontinuities. Although the macroscopic assessment of ablation lines is not very sensitive, studies of RF in models in vitro have demonstrated that the contour of the zone of ablation, which is visually appreciable as discolored tissue, can be useful as a marker of lesion because it corresponds to the isothermal line of 60°C.51 The present protocols of intraoperative endocardial RF usually foresee a program of 70-85°C.16,32 Nonetheless, to guarantee the effectiveness of the lesion it is necessary to carry out intraoperative validation, that is, to confirm the degree of blockade of electrical conduction. At this time we used intraoperative energy protocols without confirming in situ the effectiveness of the ablation lines. A continuous lesion may not be effective if it is not transmural. In contrast, a discontinuous ablation line with gaps of less than 2-3 mm can be effective if it is transmural.52,53 In a canine model of RF, Avitall et al demonstrated that impedance is the best parameter for controlling the depth of the lesion during ablation, whereas temperature is the ideal parameter for controlling the width of the lesion.54 At present, most available devices perform ablation with temperature control. In daily practice, we think that the macroscopic appearance of the ablation line can also be used to check ablation until conduction through the ablation line can be confirmed intraoperatively. Nonetheless, no bibliographic references confirm this opinion.
The incidence of postoperative atrial arrhythmias is one of the main problems of the maze procedure with RF. At present, 40% to 60% of patients with mitral valve disease present recurrence of AF after a left maze procedure with RF in the first postoperative month, figures that improve throughout the following 3 months, when sinus rhythm is attained by 50-77% of patients, probably as a result of the maturation of ablation lines.14,16,32,33 Several factors seem to be involved in the early incidence of arrhythmias after RF, some related to the technique, such as defects in the transmurality of the ablation line or gaps in its continuity that allow macroreentry to occur later.33 We also assume that more generic factors of surgery must have an influence, such as the inflammatory process of surgical trauma (atriotomy, post-ischemic tissue edema, pericarditis, ect.) or the greater postoperative adrenergic tone. In our experience, 80% of patients present some type of arrhythmia, although atrial fibrillation or flutter only recurred in 4 patients. Three of these patients had AF in the surface ECG, although the atriogram revealed a pattern of regular arrhythmia in all of them that could correspond to AF with regular activity, atrial flutter, or atrial tachycardia. In our experience, the biatrial pattern of ablation lines has reduced this initial rate of recurrence of chronic AF.
The recovery of atrial contraction is another objective of AF surgery. However, maze atriotomies produce deleterious effects on atrial contraction,55 as has also been observed with RF.32 Sueda et al have communicated, in patients undergoing surgery for left mitral valve disease and left maze who recover sinus rhythm, right atrial contraction in 100% and left atrial contraction in 60%.38 Melo et al describe in 25 patients with left mitral surgery and RF maze procedure, after 6 months of follow-up, a recovery of right atrial contraction in 42% and left atrial contraction in 30% of cases.32 In our series of 10 patients, we observed an early recovery (in the first 3 months) of left and right atrial contraction in a single patient (10%), and recovery limited to the right atrium in 3 patients (30%).
Atrial size is a predictive factor in most surgical procedures for AF, which is why various surgical groups have proposed the reduction of atrial tissue.10,25,27 Melo et al report a recurrence rate of AF of 80% at 6 months of follow-up after RF isolation of the pulmonary veins among patients with mitral valve disease and atria of more than 200 ml in volume, measured by surgical balloon.32 In our series the follow-up was shorter, but most of the patients had atrial enlargement in echocardiography, which suggests a priori less favorable results of AF surgery. We normalized atrial size in only one patient, due to compression of neighboring structures.
This study has various limitations, such as a small number of patients, a short period of follow-up for a type of surgery that requires longer term results, and results influenced by the antiarrhythmic effect of amiodarone. Another limitation has been the lack of atrial electrophysiology studies in postoperative arrhythmias, which are fundamental for differentiating the various electrophysiological mechanisms involved. Our main goal in this study has been to describe the initial results obtained with this new surgical technique, for which there is little experience in the references, which could constitute yet another step forward in the surgical treatment of AF. Comparative studies with other surgical procedures will be necessary to confirm these results. Our surgical group is currently pursuing this line of endeavor, although the introduction of new concepts in the treatment of AF will probably cause us to change our work protocols.
Our initial experience with the application of mixed RF from both the endocardium and epicardium to compartmentalize both atria has shown satisfactory results without increasing surgical risk. The initial effectiveness of the treatment of AF has been 80%. Intraoperative RF has allowed us to reproduce surgical atriotomy simply and safely. Right atrial epicardial ablation can be performed in all patients without extracorporeal circulation. Inspection of the macroscopic appearance of the ablation lines is also important during intraoperative RF application, although not sufficient to validate the ablation. Although at the time of release no patient presented recurrence of permanent AF, postoperative arrhythmias have been the most noteworthy problem.
Correspondencia: Dr. F. Hornero Sos.
Servicio de Cirugía Cardíaca.
Hospital General Universitario de Valencia.
Avda. Tres Cruces, s/n. 46014 Valencia. Spain
E-mail: hornero_fer@gva.es